Lab PI: Mary Linton B. Peters, MD, MS, FACP
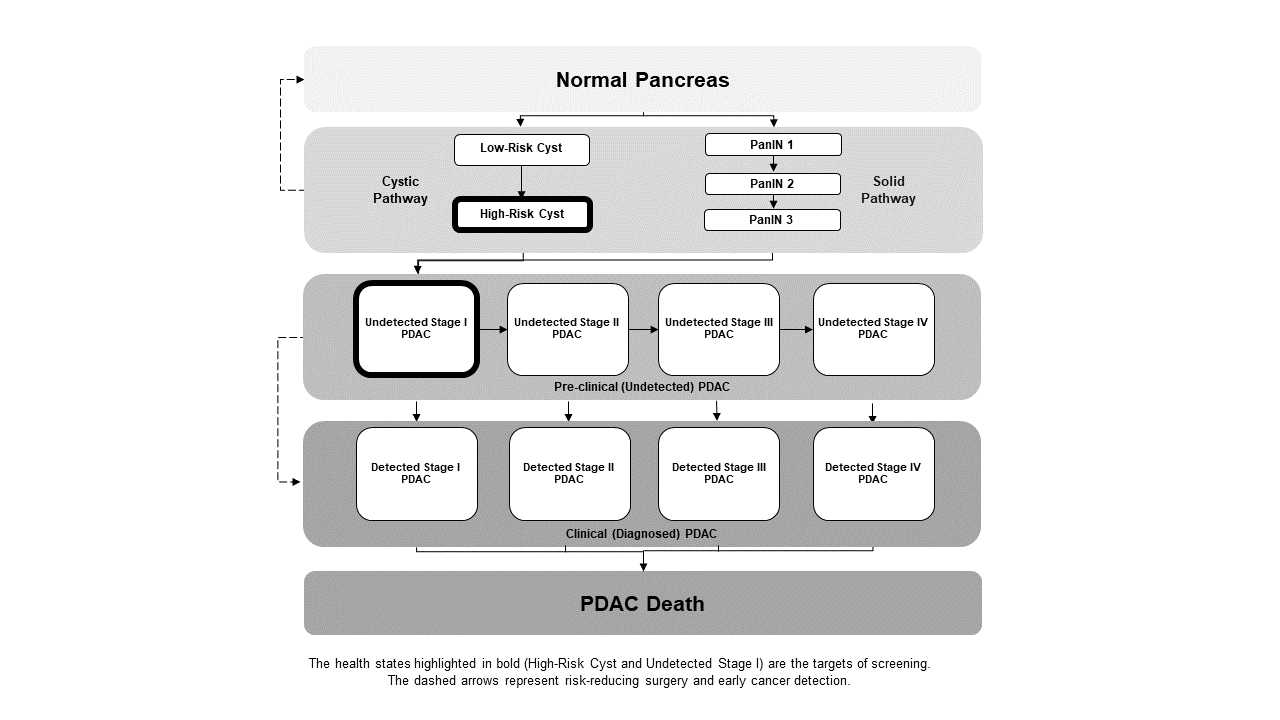
Pancreatic adenocarcinoma is a highly lethal malignancy, most often diagnosed at a late stage when it is no longer surgically resectable. There is significant interest in methods of earlier detection or prevention to increase survival, but unfortunately, pancreatic cancer risk factors have been challenging to identify. Our team has developed a microsimulation model of the development of pancreatic cancer from both cystic and solid pre-cancerous states (see figure below). We use this model to ask research questions including:
- How can we use germline genetic testing to identify high-risk family members who would benefit from screening?
- When should screening for pancreatic cancer start, and how often should it be performed, for people at various risk of the disease?
- How cost-effective is screening for pancreatic cancer?
2024
Peters, Mary Linton B; Eckel, Andrew; Seguin, Claudia L; Davidi, Barak; Howard, David H; Knudsen, Amy B; Pandharipande, Pari V
Cost-Effectiveness Analysis of Screening for Pancreatic Cancer Among High-Risk Populations Journal Article
In: JCO Oncol Pract, vol. 20, iss. 2, pp. 278-290, 2024, ISSN: 2688-1535.
@article{pmid38086003,
title = {Cost-Effectiveness Analysis of Screening for Pancreatic Cancer Among High-Risk Populations},
author = {Mary Linton B Peters and Andrew Eckel and Claudia L Seguin and Barak Davidi and David H Howard and Amy B Knudsen and Pari V Pandharipande},
doi = {10.1200/OP.23.00495},
issn = {2688-1535},
year = {2024},
date = {2024-02-01},
urldate = {2024-02-01},
journal = {JCO Oncol Pract},
volume = {20},
issue = {2},
pages = {278-290},
abstract = {PURPOSE: We evaluated the potential cost-effectiveness of combined magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) screening for pancreatic ductal adenocarcinoma (PDAC) among populations at high risk for the disease.nnMETHODS: We used a microsimulation model of the natural history of PDAC to estimate the lifetime health benefits, costs, and cost-effectiveness of PDAC screening among populations with specific genetic risk factors for PDAC, including and , , , Lynch syndrome, , , and . For each high-risk population, we simulated 29 screening strategies, defined by starting age and frequency. Screening included MRI with follow-up EUS in a subset of patients. Costs of tests were based on Medicare reimbursement for MRI, EUS, fine-needle aspiration biopsy, and pancreatectomy. Cancer-related cost by stage of disease and phase of treatment was based on the literature. For each high-risk population, we performed an incremental cost-effectiveness analysis, assuming a willingness-to-pay (WTP) threshold of $100,000 US dollars (USD) per quality-adjusted life year (QALY) gained.nnRESULTS: For men with relative risk (RR) 12.33 () and RR 28 (), annual screening was cost-effective, starting at age 55 and 40 years, respectively. For women, screening was only cost-effective for those with RR 28 (), with annual screening starting at age 45 years.nnCONCLUSION: Combined MRI/EUS screening may be a cost-effective approach for the highest-risk populations (among mutations considered, those with RR \>12). However, for those with moderate risk (RR, 5-12), screening would only be cost-effective at higher WTP thresholds (eg, $200K USD/QALY) or with once-only screening.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2022
Peters, Mary Linton B.; Eckel, Andrew; Lietz, Anna; Seguin, Claudia; Mueller, Peter; Hur, Chin; Pandharipande, Pari V.
In: Pancreatology, vol. 22, iss. 6, pp. 760-769, 2022, ISSN: 1424-3903.
@article{PETERS2022,
title = {Genetic testing to guide screening for pancreatic ductal adenocarcinoma: Results of a microsimulation model},
author = {Mary Linton B. Peters and Andrew Eckel and Anna Lietz and Claudia Seguin and Peter Mueller and Chin Hur and Pari V. Pandharipande},
url = {https://www.sciencedirect.com/science/article/pii/S1424390322001703},
doi = {https://doi.org/10.1016/j.pan.2022.05.003},
issn = {1424-3903},
year = {2022},
date = {2022-09-22},
urldate = {2022-05-31},
journal = {Pancreatology},
volume = {22},
issue = {6},
pages = {760-769},
abstract = {Background
First-degree relatives (FDRs) of patients with pancreatic ductal adenocarcinoma (PDAC) have elevated PDAC risk, partially due to germline genetic variants. We evaluated the potential effectiveness of genetic testing to target MRI-based screening among FDRs.
Methods
We used a microsimulation model of PDAC, calibrated to Surveillance, Epidemiology, and End Results (SEER) data, to estimate the potential life expectancy (LE) gain of screening for each of the following groups of FDRs: indviduals who test positive for each of eight variants associated with elevated PDAC risk (e.g., BRCA2, CDKN2A); individuals who test negative; and individuals who do not test. Screening was assumed to take place if LE gains were achievable. We simulated multiple screening approaches, defined by starting age and frequency. Sensitivity analysis evaluated changes in results given varying model assumptions.
Results
For women, 92% of mutation carriers had projected LE gains from screening for PDAC, if screening strategies (start age, frequency) were optimized. Among carriers, LE gains ranged from 0.1 days (ATM + women screened once at age 70) to 510 days (STK11+ women screened annually from age 40). For men, LE gains were projected for all mutation carriers, ranging from 0.2 days (BRCA1+ men screened once at age 70) to 620 days (STK11+ men screened annually from age 40). For men and women who did not undergo genetic testing, or for whom testing showed no variant, screening yielded small LE benefit (0\textendash2.1 days).
Conclusions
Genetic testing of FDRs can inform targeted PDAC screening by identifying which FDRs may benefit.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
First-degree relatives (FDRs) of patients with pancreatic ductal adenocarcinoma (PDAC) have elevated PDAC risk, partially due to germline genetic variants. We evaluated the potential effectiveness of genetic testing to target MRI-based screening among FDRs.
Methods
We used a microsimulation model of PDAC, calibrated to Surveillance, Epidemiology, and End Results (SEER) data, to estimate the potential life expectancy (LE) gain of screening for each of the following groups of FDRs: indviduals who test positive for each of eight variants associated with elevated PDAC risk (e.g., BRCA2, CDKN2A); individuals who test negative; and individuals who do not test. Screening was assumed to take place if LE gains were achievable. We simulated multiple screening approaches, defined by starting age and frequency. Sensitivity analysis evaluated changes in results given varying model assumptions.
Results
For women, 92% of mutation carriers had projected LE gains from screening for PDAC, if screening strategies (start age, frequency) were optimized. Among carriers, LE gains ranged from 0.1 days (ATM + women screened once at age 70) to 510 days (STK11+ women screened annually from age 40). For men, LE gains were projected for all mutation carriers, ranging from 0.2 days (BRCA1+ men screened once at age 70) to 620 days (STK11+ men screened annually from age 40). For men and women who did not undergo genetic testing, or for whom testing showed no variant, screening yielded small LE benefit (0–2.1 days).
Conclusions
Genetic testing of FDRs can inform targeted PDAC screening by identifying which FDRs may benefit.
2019
Weaver, Davis; Lietz, Anna; Mercaldo, Sarah Fletcher; Peters, Mary Linton B.; Hur, Chin; Kong, Chung Yin; Wolpin, Brian M; Megibow, Alec J; Berland, Lincoln L; Knudsen, Amy; Pandharipande, Pari
In: AJR. American journal of roentgenology, vol. 212, no. 3, pp. 596-601, 2019, ISSN: 1546-3141.
@article{Weaver2019,
title = {Testing for Verification Bias in Reported Malignancy Risks for Side-Branch Intraductal Papillary Mucinous Neoplasms: A Simulation Modeling Approach.},
author = {Davis Weaver and Anna Lietz and Sarah Fletcher Mercaldo and Mary Linton B. Peters and Chin Hur and Chung Yin Kong and Brian M Wolpin and Alec J Megibow and Lincoln L Berland and Amy Knudsen and Pari Pandharipande},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30620679},
doi = {10.2214/AJR.18.20180},
issn = {1546-3141},
year = {2019},
date = {2019-03-01},
urldate = {2019-03-01},
journal = {AJR. American journal of roentgenology},
volume = {212},
number = {3},
pages = {596-601},
abstract = {The objective of our study was to test for the possibility that published malignancy risks for side-branch intraductal papillary mucinous neoplasms (IPMNs) are overestimates, likely due to verification bias. We tested for possible verification bias using simulation modeling techniques. First, in age-defined hypothetical cohorts of 10 million persons, we projected the frequency of pancreatic ductal adenocarcinoma (PDAC) arising from side-branch IPMNs over 5 years using published estimates of their prevalence (4.4%) and rate of malignant transformation (1.9%). Second, we projected the total number of PDAC cases in corresponding cohorts over the same time horizon using national cancer registry data. For each cohort, we determined whether the percentage of all PDAC cases that arose from side-branch IPMNs (i.e., side-branch IPMN-associated PDAC cases) was clinically plausible using an upper limit of 10% to define plausibility, as estimated from the literature. Model assumptions and parameter uncertainty were evaluated in sensitivity analysis. Across all cohorts, percentages of side-branch IPMN-associated PDACs greatly exceeded 10%. In the base case (mean age = 55.7 years), 80% of PDAC cases arose from side-branch IPMNs (7877/9786). In the oldest cohort evaluated (mean age = 75 years), this estimate was 76% (14,227/18,714). In a secondary analysis, we found that if an upper limit threshold of 10% for side-branch IPMN-associated PDAC was imposed, the model-predicted rate of malignancy for side-branch IPMNs would be less than 0.24% over a 5-year time horizon, substantially lower than most literature-based estimates. Our results suggest that reported malignancy risks associated with side-branch IPMNs are likely to be overestimates and imply the presence of verification bias.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2018
Peters, Mary Linton B.; Eckel, Andrew; Mueller, Peter P.; Tramontano, Angela; Weaver, Davis; Lietz, Anna; Hur, Chin; Kong, Chung Yin; Pandharipande, Pari
Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: Results of a simulation model. Journal Article
In: Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.], vol. 18, no. 8, pp. 928-934, 2018, ISSN: 1424-3911.
@article{Peters2018,
title = {Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: Results of a simulation model.},
author = {Mary Linton B. Peters and Andrew Eckel and Peter P. Mueller and Angela Tramontano and Davis Weaver and Anna Lietz and Chin Hur and Chung Yin Kong and Pari Pandharipande},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30143405},
doi = {10.1016/j.pan.2018.07.009},
issn = {1424-3911},
year = {2018},
date = {2018-12-01},
urldate = {2018-12-01},
journal = {Pancreatology : official journal of the International Association of Pancreatology (IAP) ... [et al.]},
volume = {18},
number = {8},
pages = {928-934},
abstract = {To gain insight into the natural history and carcinogenesis pathway of Pancreatic Intraepithelial Neoplasia (PanIN) lesions by building a calibrated simulation model of PanIN progression to pancreatic ductal adenocarcinoma (PDAC) METHODS: We revised a previously validated simulation model of solid PDAC, calibrating the model to fit data from the National Cancer Institute's Surveillance, Epidemiology, and End Results program and published literature on PanIN prevalence by age. We estimated the likelihood of progression from PanIN states (1, 2, and 3) to PDAC and the time between PanIN onset and PDAC (dwell time). We evaluated a hypothetical intervention to test for and treat PanIN 3 lesions to estimate the potential benefits from PanIN detection. We estimated the lifetime probability of progressing from PanIN 1 to PDAC to be 1.5% (men), 1.3% (women). Progression from PanIN 1 to PDAC took 33.6 years and 35.3 years, respectively, and from PanIN 3 to PDAC took 11.3 years and 12.3 years. A hypothetical test for PanIN 3 detection and treatment could provide a maximum, average life expectancy gain of 40 days. Our modeling analysis estimates PanINs have a relatively indolent course to PDAC, supporting the feasibility of potential future early detection strategies.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2015
Pandharipande, Pari; Jeon, Alvin; Heberle, Curtis; Dowling, Emily; Kong, Chung Yin; Chung, Daniel C.; Brugge, William R.; Hur, Chin
Screening for Pancreatic Adenocarcinoma in BRCA2 Mutation Carriers: Results of a Disease Simulation Model Journal Article
In: EBioMedicine, vol. 2, no. 12, pp. 1980–1986, 2015.
@article{Pandharipande2015a,
title = {Screening for Pancreatic Adenocarcinoma in BRCA2 Mutation Carriers: Results of a Disease Simulation Model},
author = {Pari Pandharipande and Alvin Jeon and Curtis Heberle and Emily Dowling and Chung Yin Kong and Daniel C. Chung and William R. Brugge and Chin Hur},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26844277},
doi = {10.1016/j.ebiom.2015.11.005},
year = {2015},
date = {2015-11-01},
urldate = {2015-11-01},
journal = {EBioMedicine},
volume = {2},
number = {12},
pages = {1980--1986},
institution = {Institute for Technology Assessment, Massachusetts General Hospital, Boston, USA; Gastroenterology Division, Massachusetts General Hospital, Boston, USA; Harvard Medical School, Boston, USA.},
abstract = {BRCA2 mutation carriers are at increased risk for multiple cancers including pancreatic adenocarcinoma (PAC). Our goal was to compare the effectiveness of different PAC screening strategies in BRCA2 mutation carriers, from the standpoint of life expectancy.A previously published Markov model of PAC was updated and extended to incorporate key aspects of BRCA2 mutation carrier status, including competing risks of breast- and ovarian-cancer specific mortality. BRCA2 mutation carriers were modeled and analyzed as the primary cohort for the analysis. Additional higher risk BRCA2 cohorts that were stratified according to the number of first-degree relatives (FDRs) with PAC were also analyzed. For each cohort, one-time screening and annual screening were evaluated, with screening starting at age 50 in both strategies. The primary outcome was net gain in life expectancy (LE) compared to no screening. Sensitivity analysis was performed on key model parameters, including surgical mortality and MRI test performance.One-time screening at age 50 resulted in a LE gain of 3.9 days for the primary BRCA2 cohort, and a gain of 5.8 days for those with BRCA2 and one FDR. Annual screening resulted in LE loss of 12.9 days for the primary cohort and 1.3 days for BRCA2 carriers with 1 FDR, but resulted in 20.6 days gained for carriers with 2 FDRs and 260 days gained for those with 3 FDRs. For patients with ≥ 3 FDRs, annual screening starting at an earlier age (i.e. 35-40) was optimal.Among BRCA2 mutation carriers, aggressive screening regimens may be ineffective unless additional indicators of elevated risk (e.g., 2 or more FDRs) are present. More clinical studies are needed to confirm these findings.American Cancer Society - New England Division - Ellison Foundation Research Scholar Grant (RSG-15-129-01-CPHPS).},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2014
Pandharipande, Pari; Heberle, Curtis; Dowling, Emily; Kong, Chung Yin; Tramontano, Angela; Perzan, Katherine; Brugge, William; Hur, Chin
Targeted Screening of Individuals at High Risk for Pancreatic Cancer: Results of a Simulation Model Journal Article
In: Radiology, vol. 275, no. 1, pp. 177-87, 2014.
@article{Pandharipande2014,
title = {Targeted Screening of Individuals at High Risk for Pancreatic Cancer: Results of a Simulation Model},
author = {Pari Pandharipande and Curtis Heberle and Emily Dowling and Chung Yin Kong and Angela Tramontano and Katherine Perzan and William Brugge and Chin Hur},
url = {http://www.ncbi.nlm.nih.gov/pubmed/25393849},
doi = {10.1148/radiol.14141282},
year = {2014},
date = {2014-04-01},
urldate = {2014-04-01},
journal = {Radiology},
volume = {275},
number = {1},
pages = {177-87},
institution = {From the Massachusetts General Hospital Institute for Technology Assessment (P.V.P., C.H., E.C.D., C.Y.K., A.T., K.E.P., C.H.), Department of Radiology (P.V.P., C.H., E.C.D., C.Y.K., A.T.), and Department of General Medicine, Gastrointestinal Unit (},
abstract = {Purpose To identify when, from the standpoint of relative risk, magnetic
resonance (MR) imaging-based screening may be effective in patients
with a known or suspected genetic predisposition to pancreatic cancer.
Materials and Methods The authors developed a Markov model of pancreatic
ductal adenocarcinoma ( PDAC pancreatic ductal adenocarcinoma ).
The model was calibrated to National Cancer Institute Surveillance,
Epidemiology, and End Results registry data and informed by the literature.
A hypothetical screening strategy was evaluated in which all population
individuals underwent one-time MR imaging screening at age 50 years.
Screening outcomes for individuals with an average risk for PDAC
pancreatic ductal adenocarcinoma ("base case") were compared with
those for individuals at an increased risk to assess for differential
benefits in populations with a known or suspected genetic predisposition.
Effects of varying key inputs, including MR imaging performance,
surgical mortality, and screening age, were evaluated with a sensitivity
analysis. Results In the base case, screening resulted in a small
number of cancer deaths averted (39 of 100 000 men, 38 of 100 000
women) and a net decrease in life expectancy (-3 days for men, -4
days for women), which was driven by unnecessary pancreatic surgeries
associated with false-positive results. Life expectancy gains were
achieved if an individual's risk for PDAC pancreatic ductal adenocarcinoma
exceeded 2.4 (men) or 2.7 (women) times that of the general population.
When relative risk increased further, for example to 30 times that
of the general population, averted cancer deaths and life expectancy
gains increased substantially (1219 of 100 000 men, life expectancy
gain: 65 days; 1204 of 100 000 women, life expectancy gain: 71 days).
In addition, results were sensitive to MR imaging specificity and
the surgical mortality rate. Conclusion Although PDAC pancreatic
ductal adenocarcinoma screening with MR imaging for the entire population
is not effective, individuals with even modestly increased risk may
benefit. © RSNA, 2014 Online supplemental material is available for
this article.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
resonance (MR) imaging-based screening may be effective in patients
with a known or suspected genetic predisposition to pancreatic cancer.
Materials and Methods The authors developed a Markov model of pancreatic
ductal adenocarcinoma ( PDAC pancreatic ductal adenocarcinoma ).
The model was calibrated to National Cancer Institute Surveillance,
Epidemiology, and End Results registry data and informed by the literature.
A hypothetical screening strategy was evaluated in which all population
individuals underwent one-time MR imaging screening at age 50 years.
Screening outcomes for individuals with an average risk for PDAC
pancreatic ductal adenocarcinoma ("base case") were compared with
those for individuals at an increased risk to assess for differential
benefits in populations with a known or suspected genetic predisposition.
Effects of varying key inputs, including MR imaging performance,
surgical mortality, and screening age, were evaluated with a sensitivity
analysis. Results In the base case, screening resulted in a small
number of cancer deaths averted (39 of 100 000 men, 38 of 100 000
women) and a net decrease in life expectancy (-3 days for men, -4
days for women), which was driven by unnecessary pancreatic surgeries
associated with false-positive results. Life expectancy gains were
achieved if an individual's risk for PDAC pancreatic ductal adenocarcinoma
exceeded 2.4 (men) or 2.7 (women) times that of the general population.
When relative risk increased further, for example to 30 times that
of the general population, averted cancer deaths and life expectancy
gains increased substantially (1219 of 100 000 men, life expectancy
gain: 65 days; 1204 of 100 000 women, life expectancy gain: 71 days).
In addition, results were sensitive to MR imaging specificity and
the surgical mortality rate. Conclusion Although PDAC pancreatic
ductal adenocarcinoma screening with MR imaging for the entire population
is not effective, individuals with even modestly increased risk may
benefit. © RSNA, 2014 Online supplemental material is available for
this article.




