Colorectal cancer is the second leading cause of cancer death in the United States. Randomized trials have shown that routine screening for colorectal cancer can reduce both the incidence of and the mortality from the disease. However, due to practical limitations, randomized trials cannot be performed to address all questions about colorectal cancer screening. Accordingly, we have developed a microsimulation model – the Simulation Model of Colorectal Cancer (SimCRC) – that can be used to estimate outcomes under different screening scenarios.
SimCRC is one of three models that comprise the National Cancer Institute-funded Cancer Intervention and Surveillance Modeling Network (CISNET) consortium. Through CISNET, SimCRC has been used to inform several national policy and Medicare coverage decisions surrounding colorectal cancer screening:
- 2021 (Knudsen et al.), 2016 (Knudsen and Zauber, et al.), and 2008 (Zauber et al.) US Preventive Services Task Force Recommendations for Colorectal Cancer Screening
- 2018 American Cancer Society Colorectal Cancer Screening Guidelines (Meester et al.)
- Centers for Medicare and Medicaid Services National Coverage Determinations for stood DNA testing (Landsorp_Vogelaar et al., Naber and Knudsen, et al.) and computed tomography colonography (Knudsen et al.)
Of note, Dr. Knudsen’s team led CISNET analyses that helped to inform the US Preventive Task Force’s 2021 recommendation to begin colorectal cancer screening at age 45. The CISNET team developed an interactive tool for viewing model outcomes by the ages to begin and end screening.
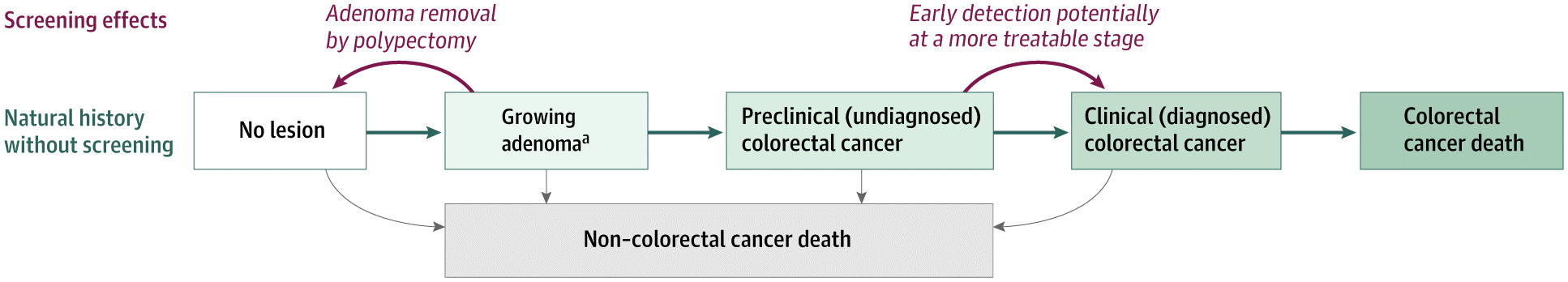
Model schematic of the natural history of colorectal cancer and the effects of screening as simulated by the Simulation Model of ColoRectal Cancer (SimCRC).
2021
Knudsen, Amy; Rutter, Carolyn M; Meester, Reinier G S; Lansdorp-Vogelaar, Iris; Zauber, Ann G; Kuntz, Karen M
Colorectal Cancer Screening in Young Adults. Journal Article
In: Annals of internal medicine, vol. 174, no. 7, pp. 1039–1040, 2021, ISSN: 1539-3704, ().
@article{Knudsen2021a,
title = {Colorectal Cancer Screening in Young Adults.},
author = {Amy Knudsen and Carolyn M Rutter and Reinier G S Meester and Iris Lansdorp-Vogelaar and Ann G Zauber and Karen M Kuntz},
url = {https://pubmed.ncbi.nlm.nih.gov/34280341/},
doi = {10.7326/L21-0245},
issn = {1539-3704},
year = {2021},
date = {2021-07-01},
journal = {Annals of internal medicine},
volume = {174},
number = {7},
pages = {1039--1040},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Knudsen, Amy; Rutter, Carolyn M.; Peterse, Elisabeth F. P.; Lietz, Anna; Seguin, Claudia; Meester, Reinier G. S.; Perdue, Leslie A.; Lin, Jennifer S.; Siegel, Rebecca L.; Doria-Rose, V. Paul; Feuer, Eric J.; Zauber, Ann G.; Kuntz, Karen M.; Lansdorp-Vogelaar, Iris
Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force Journal Article
In: JAMA, vol. 325, no. 19, pp. 1998-2011, 2021, ISSN: 0098-7484, ().
@article{knudsen2021,
title = {Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force},
author = {Amy Knudsen and Carolyn M. Rutter and Elisabeth F. P. Peterse and Anna Lietz and Claudia Seguin and Reinier G. S. Meester and Leslie A. Perdue and Jennifer S. Lin and Rebecca L. Siegel and V. Paul Doria-Rose and Eric J. Feuer and Ann G. Zauber and Karen M. Kuntz and Iris Lansdorp-Vogelaar},
url = {https://doi.org/10.1001/jama.2021.5746},
doi = {10.1001/jama.2021.5746},
issn = {0098-7484},
year = {2021},
date = {2021-05-01},
journal = {JAMA},
volume = {325},
number = {19},
pages = {1998-2011},
abstract = {The US Preventive Services Task Force (USPSTF) is updating its 2016 colorectal cancer screening recommendations.To provide updated model-based estimates of the benefits, burden, and harms of colorectal cancer screening strategies and to identify strategies that may provide an efficient balance of life-years gained (LYG) from screening and colonoscopy burden to inform the USPSTF.Comparative modeling study using 3 microsimulation models of colorectal cancer screening in a hypothetical cohort of 40-year-old US individuals at average risk of colorectal cancer.Screening from ages 45, 50, or 55 years to ages 70, 75, 80, or 85 years with fecal immunochemical testing (FIT), multitarget stool DNA testing, flexible sigmoidoscopy alone or with FIT, computed tomography colonography, or colonoscopy. All persons with an abnormal noncolonoscopy screening test result were assumed to undergo follow-up colonoscopy. Screening intervals varied by test. Full adherence with all procedures was assumed.Estimated LYG relative to no screening (benefit), lifetime number of colonoscopies (burden), number of complications from screening (harms), and balance of incremental burden and benefit (efficiency ratios). Efficient strategies were those estimated to require fewer additional colonoscopies per additional LYG relative to other strategies.Estimated LYG from screening strategies ranged from 171 to 381 per 1000 40-year-olds. Lifetime colonoscopy burden ranged from 624 to 6817 per 1000 individuals, and screening complications ranged from 5 to 22 per 1000 individuals. Among the 49 strategies that were efficient options with all 3 models, 41 specified screening beginning at age 45. No single age to end screening was predominant among the efficient strategies, although the additional LYG from continuing screening after age 75 were generally small. With the exception of a 5-year interval for computed tomography colonography, no screening interval predominated among the efficient strategies for each modality. Among the strategies highlighted in the 2016 USPSTF recommendation, lowering the age to begin screening from 50 to 45 years was estimated to result in 22 to 27 additional LYG, 161 to 784 additional colonoscopies, and 0.1 to 2 additional complications per 1000 persons (ranges are across screening strategies, based on mean estimates across models). Assuming full adherence, screening outcomes and efficient strategies were similar by sex and race and across 3 scenarios for population risk of colorectal cancer.This microsimulation modeling analysis suggests that screening for colorectal cancer with stool tests, endoscopic tests, or computed tomography colonography starting at age 45 years provides an efficient balance of colonoscopy burden and life-years gained.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Peterse, Elisabeth F. P.; Meester, Reinier G. S.; Jonge, Lucie; Omidvari, Amir-Houshang; Alarid-Escudero, Fernando; Knudsen, Amy; Zauber, Ann G.; Lansdorp-Vogelaar, Iris
Comparing the cost-effectiveness of innovative colorectal cancer screening tests. Journal Article
In: Journal of the National Cancer Institute, vol. 113, no. 2, pp. 154-161, 2021, ISSN: 1460-2105, ().
@article{Peterse2020,
title = {Comparing the cost-effectiveness of innovative colorectal cancer screening tests.},
author = {Elisabeth F. P. Peterse and Reinier G. S. Meester and Lucie Jonge and Amir-Houshang Omidvari and Fernando Alarid-Escudero and Amy Knudsen and Ann G. Zauber and Iris Lansdorp-Vogelaar},
url = {https://pubmed.ncbi.nlm.nih.gov/32761199/},
doi = {10.1093/jnci/djaa103},
issn = {1460-2105},
year = {2021},
date = {2021-02-01},
journal = {Journal of the National Cancer Institute},
volume = {113},
number = {2},
pages = {154-161},
abstract = {Colorectal cancer (CRC) screening with colonoscopy and the fecal immunochemical test (FIT) is underutilized. Innovative tests could increase screening acceptance. This study determined which of the available alternatives is most promising from a cost-effectiveness perspective. The previously-validated MISCAN-Colon model was used to evaluate the cost-effectiveness of screening with capsule endoscopy every 5 or 10 years, computed tomographic colonography (CTC) every 5 years, the multi-target stool DNA (mtSDNA) test every 1 or 3 years, and the methylated SEPT9 DNA plasma assay (mSEPT9) every 1 or 2 years. We also compared these strategies to annual FIT screening and colonoscopy screening every 10 years. Quality-adjusted life-years gained (QALYG), number of colonoscopies, and incremental cost-effectiveness ratios (ICERs) were projected. We assumed a willingness-to-pay threshold of $100,000 per QALYG. Among the alternative tests, CTC every 5 years, annual mSEPT9 and annual mtSDNA screening had ICERs of $1,092, $63,253 and $214,974 per QALYG, respectively. Other screening strategies were more costly and less effective than (a combination of) these three. Under the assumption of perfect adherence, annual mSEPT9 screening resulted in more QALYG, CRC cases averted and CRC deaths averted than annual FIT screening, but led to a high rate of colonoscopy referral (51% after 3 years, 69% after 5 years). The alternative tests were not cost-effective compared to FIT and colonoscopy. This study suggests that for individuals not willing to participate in FIT or colonoscopy screening, mSEPT9 is the test of choice if the high colonoscopy referral rate is acceptable to them.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rutter, Carolyn M.; Knudsen, Amy; Lin, Jennifer S.; Bouskill, Kathryn E.
Black and White Differences in Colorectal Cancer Screening and Screening Outcomes: A Narrative Review. Journal Article
In: Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, vol. 30, no. 1, pp. 3-12, 2021, ISSN: 1538-7755, ().
@article{Rutter2020,
title = {Black and White Differences in Colorectal Cancer Screening and Screening Outcomes: A Narrative Review.},
author = {Carolyn M. Rutter and Amy Knudsen and Jennifer S. Lin and Kathryn E. Bouskill},
url = {https://pubmed.ncbi.nlm.nih.gov/33144285/},
doi = {10.1158/1055-9965.EPI-19-1537},
issn = {1538-7755},
year = {2021},
date = {2021-01-01},
journal = {Cancer epidemiology, biomarkers \& prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology},
volume = {30},
number = {1},
pages = {3-12},
abstract = {Racial disparities in colorectal cancer (CRC) incidence are widely documented. There are two potential mechanisms for these disparities: differences in access to screening, including screening follow-up, and differences in underlying risk of CRC. We reviewed the literature for evidence of these two mechanisms. We show that higher CRC incidence in blacks relative to whites emerged only after the dissemination of screening and describe evidence of racial disparities in screening rates. In contrast to the strong evidence for differences in CRC screening utilization, there is limited evidence for racial differences in adenoma prevalence. In general, black and white patients who are screened have similar adenoma prevalence, though there is some evidence that advanced adenomas and adenomas in the proximal colon are somewhat more likely in black than white patients. We conclude that higher rates of CRC incidence among black patients are primarily driven by lower rates of CRC screening. Our findings highlight the need to increase black patients' access to quality screening to reduce CRC incidence and mortality.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Seguin, Claudia; Lietz, Anna; Wright, Jason D.; Wright, Alexi; Knudsen, Amy; Pandharipande, Pari
Surveillance in Older Women With Incidental Ovarian Cysts: Maximal Projected Benefits by Age and Comorbidity Level. Journal Article
In: Journal of the American College of Radiology : JACR, vol. 18, no. 1 PT A, pp. 10-18, 2021, ISSN: 1558-349X, ().
@article{Seguin2020,
title = {Surveillance in Older Women With Incidental Ovarian Cysts: Maximal Projected Benefits by Age and Comorbidity Level.},
author = {Claudia Seguin and Anna Lietz and Jason D. Wright and Alexi Wright and Amy Knudsen and Pari Pandharipande},
url = {https://pubmed.ncbi.nlm.nih.gov/33096089/},
doi = {10.1016/j.jacr.2020.09.048},
issn = {1558-349X},
year = {2021},
date = {2021-01-01},
journal = {Journal of the American College of Radiology : JACR},
volume = {18},
number = {1 PT A},
pages = {10-18},
abstract = {The aim of this study was to estimate effects on life expectancy (LE) of imaging-based ovarian surveillance after detection of incidental postmenopausal ovarian cysts, under different assumptions of patient age, comorbidity level, and cancer risk and detection. A decision-analytic Markov model was developed to estimate LE benefits. Hypothetical cohorts of postmenopausal women with simple ovarian cysts were evaluated, with varied age (66-80 years) and comorbidity level (none, mild, moderate, severe). For each cohort, imaging "follow-up" (2 years) and "no-follow-up" strategies were compared. Consistent with current evidence, increased cancer risk in patients with cysts was not assumed; however, incident ovarian cancers could be detected during follow-up. To estimate theoretical maximal LE gains from follow-up, perfect ovarian cancer detection and treatment during follow-up were assumed. This and other key assumptions were varied in sensitivity analysis. Projected LE gains from follow-up were limited. For 66-, 70-, 75-, and 80-year-old women with no comorbidities, LE gains were 5.1, 5.1, 4.5, and 3.7 days; with severe comorbidities, they were 3.5, 3.2, 2.7, and 2.1 days. With sensitivity of 50% for cancer detection, they were 3.7 days for 66-year-old women with no comorbidities and 1.3 days for 80-year-old women with severe comorbidities. When cancer risk for women with cysts was assumed to be elevated (1.1 times average risk), LE gains increased only modestly (5.6 and 2.3 days for analogous cohorts). Even in the circumstance of perfect ovarian cancer detection and treatment, surveillance of postmenopausal women (≥66 years of age) with simple cysts affords limited benefits, particularly in women with advanced age and comorbidities.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2020
DeYoreo, Maria; Lansdorp-Vogelaar, Iris; Knudsen, Amy; Kuntz, Karen M.; Zauber, Ann G.; Rutter, Carolyn M.
Validation of Colorectal Cancer Models on Long-Term Outcomes from a Randomized Controlled Trial. Journal Article
In: Medical decision making : an international journal of the Society for Medical Decision Making, vol. 40, no. 8, pp. 1034-1040, 2020, ISSN: 1552-681X, ().
@article{DeYoreo2020,
title = {Validation of Colorectal Cancer Models on Long-Term Outcomes from a Randomized Controlled Trial.},
author = {Maria DeYoreo and Iris Lansdorp-Vogelaar and Amy Knudsen and Karen M. Kuntz and Ann G. Zauber and Carolyn M. Rutter},
url = {https://pubmed.ncbi.nlm.nih.gov/33078673/},
doi = {10.1177/0272989X20961095},
issn = {1552-681X},
year = {2020},
date = {2020-11-01},
journal = {Medical decision making : an international journal of the Society for Medical Decision Making},
volume = {40},
number = {8},
pages = {1034-1040},
abstract = {Microsimulation models are often used to predict long-term outcomes and guide policy decisions regarding cancer screening. The United Kingdom Flexible Sigmoidoscopy Screening (UKFSS) Trial examines a one-time intervention of flexible sigmoidoscopy that was implemented before a colorectal cancer (CRC) screening program was established. Long-term study outcomes, now a full 17 y following randomization, have been published. We use the outcomes from this trial to validate 3 microsimulation models for CRC to long-term study outcomes. We find that 2 of 3 models accurately predict the relative effect of screening (the hazard ratios) on CRC-specific incidence 17 y after screening. We find that all 3 models yield predictions of the relative effect of screening on CRC incidence and mortality (i.e., the hazard ratios) that are reasonably close to the UKFSS results. Two of the 3 models accurately predict the relative reduction in CRC incidence 17 y after screening. One model accurately predicted the absolute incidence and mortality rates in the screened group. The models differ in their estimates related to adenoma detection at screening. Although high-quality screening results help to inform models, trials are expensive, last many years, and can be complicated by ethical issues and technological changes across the duration of the trial. Thus, well-calibrated and validated models are necessary to predict outcomes for which data are not available. The results from this validation demonstrate the utility of models in predicting long-term outcomes and in collaborative modeling to account for uncertainty.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Abuelo, Carolina; Ashburner, Jeffrey M.; Atlas, Steven J.; Knudsen, Amy; Morrill, James; Corona, Patricia; Shtasel, Derri; Percac-Lima, Sanja
Colorectal Cancer Screening Patient Navigation for Patients with Mental Illness and/or Substance Use Disorder: Pilot Randomized Control Trial. Journal Article
In: Journal of dual diagnosis, vol. 16, no. 4, pp. 438-446, 2020, ISSN: 1550-4271, ().
@article{Abuelo2020,
title = {Colorectal Cancer Screening Patient Navigation for Patients with Mental Illness and/or Substance Use Disorder: Pilot Randomized Control Trial.},
author = {Carolina Abuelo and Jeffrey M. Ashburner and Steven J. Atlas and Amy Knudsen and James Morrill and Patricia Corona and Derri Shtasel and Sanja Percac-Lima},
url = {https://pubmed.ncbi.nlm.nih.gov/32762637/},
doi = {10.1080/15504263.2020.1802542},
issn = {1550-4271},
year = {2020},
date = {2020-08-01},
journal = {Journal of dual diagnosis},
volume = {16},
number = {4},
pages = {438-446},
abstract = {Colorectal cancer (CRC) is the second leading cause of cancer death in the US. Screening has decreased CRC mortality. However, disadvantaged patients, particularly those with mental illness or substance use disorder (SUD), are less likely to be screened. The aim of this trial was to evaluate the impact of a patient navigation program on CRC screening in patients with mental illness and/or SUD. A pilot randomized nonblinded controlled trial was conducted from January to June 2017 in an urban community health center serving a low-income population. We randomized 251 patients aged 50-74 years with mental illness and/or SUD diagnosis overdue for CRC screening to intervention ( = 126) or usual care ( = 125) stratified by mental illness, SUD, or dual diagnosis. Intervention group patients received a letter followed by a phone call from patient navigators. Navigators helped patients overcome their individual barriers to CRC screening including: education, scheduling, explanation of bowel preparation, lack of transportation or accompaniment to appointments. If patient refused colonoscopy, navigators offered fecal occult blood testing. The main measure was proportion of patients completing CRC screening in intervention and usual care groups. Navigators contacted 85 patients (67%) in the intervention group and 26 declined to participate. In intention-to treat analysis, more patients in the intervention group received CRC screening than in the usual care group, 19% versus 10.4% ( = .04). Among 56 intervention patients who received navigation, 19 completed screening (33.9% versus 10.4% in the control group, = .001). In the subgroup of patients with SUD, 20% in the intervention group were screened compared to none in the usual care group ( = .05). A patient navigation program improved CRC screening rates in patients with mental illness and/or SUD. Larger studies in diverse care settings are needed to demonstrate generalizability and explore which modality of CRC screening is most acceptable and which navigator activities are most effective for this vulnerable population. 2016P001322.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Meester, Reinier G. S.; Lansdorp-Vogelaar, Iris; Winawer, Sidney J.; Zauber, Ann G.; Knudsen, Amy; Ladabaum, Uri
Intensity of Surveillance for Patients With Colorectal Adenomas. Journal Article
In: Annals of internal medicine, vol. 172, pp. 442, 2020, ISSN: 1539-3704, ().
@article{Meester2020a,
title = {Intensity of Surveillance for Patients With Colorectal Adenomas.},
author = {Reinier G. S. Meester and Iris Lansdorp-Vogelaar and Sidney J. Winawer and Ann G. Zauber and Amy Knudsen and Uri Ladabaum},
url = {https://pubmed.ncbi.nlm.nih.gov/32176909/},
doi = {10.7326/L19-0829},
issn = {1539-3704},
year = {2020},
date = {2020-03-01},
urldate = {2020-03-01},
journal = {Annals of internal medicine},
volume = {172},
pages = {442},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Meester, Reinier G. S.; Lansdorp-Vogelaar, Iris; Winawer, Sidney J.; Zauber, Ann G.; Knudsen, Amy; Ladabaum, Uri
Intensity of Surveillance for Patients With Colorectal Adenomas. Journal Article
In: Annals of internal medicine, vol. 172, pp. 442, 2020, ISSN: 1539-3704, ().
@article{Meester2020,
title = {Intensity of Surveillance for Patients With Colorectal Adenomas.},
author = {Reinier G. S. Meester and Iris Lansdorp-Vogelaar and Sidney J. Winawer and Ann G. Zauber and Amy Knudsen and Uri Ladabaum},
url = {https://www.ncbi.nlm.nih.gov/pubmed/32176909},
doi = {10.7326/L19-0829},
issn = {1539-3704},
year = {2020},
date = {2020-03-01},
urldate = {2020-03-01},
journal = {Annals of internal medicine},
volume = {172},
pages = {442},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2019
Meester, Reinier G S; Lansdorp-Vogelaar, Iris; Winawer, Sidney J; Zauber, Ann G; Knudsen, Amy; Ladabaum, Uri
High-Intensity Versus Low-Intensity Surveillance for Patients With Colorectal Adenomas: A Cost-Effectiveness Analysis. Journal Article
In: Annals of internal medicine, vol. 171, no. 9, pp. 612-622, 2019, ISSN: 1539-3704, ().
@article{Meester2019,
title = {High-Intensity Versus Low-Intensity Surveillance for Patients With Colorectal Adenomas: A Cost-Effectiveness Analysis.},
author = {Reinier G S Meester and Iris Lansdorp-Vogelaar and Sidney J Winawer and Ann G Zauber and Amy Knudsen and Uri Ladabaum},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31546257},
doi = {10.7326/M18-3633},
issn = {1539-3704},
year = {2019},
date = {2019-11-01},
journal = {Annals of internal medicine},
volume = {171},
number = {9},
pages = {612-622},
abstract = {Surveillance of patients with colorectal adenomas has limited long-term evidence to support current practice. To compare the lifetime benefits and costs of high- versus low-intensity surveillance. Microsimulation model. U.S. cancer registry, cost data, and published literature. U.S. patients aged 50, 60, or 70 years with low-risk adenomas (LRAs) (1 to 2 small adenomas) or high-risk adenomas (HRAs) (3 to 10 small adenomas or ≥1 large adenoma) removed after screening with colonoscopy or fecal immunochemical testing (FIT). Lifetime. Societal. No further screening or surveillance, routine screening after 10 years, low-intensity surveillance (10 years after LRA removal and 5 years after HRA removal), and high-intensity surveillance (5 years after LRA removal and 3 years after HRA removal). Colorectal cancer (CRC) incidence and incremental cost-effectiveness. Without surveillance or screening, lifetime CRC incidence for patients aged 50 years was 10.9% after LRA removal and 17.2% after HRA removal at screening colonoscopy. Subsequent colonoscopic screening, low-intensity surveillance, or high-intensity surveillance decreased incidence by 39%, 46% to 48%, and 55% to 56%, respectively. Incidence of CRC and surveillance benefits were higher for adenomas detected at FIT screening and lower for older patients. High-intensity surveillance cost less than $30 000 per quality-adjusted life-year (QALY) gained compared with low-intensity surveillance. High-intensity surveillance cost less than $100 000 per QALY gained in most alternative scenarios for adenoma recurrence, CRC incidence, longevity, quality of life, screening ages, surveillance ages, test performance, disutilities, and cost. Few surveillance outcome data exist. The model suggests that high-intensity surveillance as recommended in the United States provides modest but clinically relevant benefits over low-intensity surveillance at acceptable cost. National Cancer Institute.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Weaver, Davis; Lietz, Anna; Mercaldo, Sarah Fletcher; Peters, Mary Linton B.; Hur, Chin; Kong, Chung Yin; Wolpin, Brian M; Megibow, Alec J; Berland, Lincoln L; Knudsen, Amy; Pandharipande, Pari
In: AJR. American journal of roentgenology, vol. 212, no. 3, pp. 596-601, 2019, ISSN: 1546-3141.
@article{Weaver2019,
title = {Testing for Verification Bias in Reported Malignancy Risks for Side-Branch Intraductal Papillary Mucinous Neoplasms: A Simulation Modeling Approach.},
author = {Davis Weaver and Anna Lietz and Sarah Fletcher Mercaldo and Mary Linton B. Peters and Chin Hur and Chung Yin Kong and Brian M Wolpin and Alec J Megibow and Lincoln L Berland and Amy Knudsen and Pari Pandharipande},
url = {https://www.ncbi.nlm.nih.gov/pubmed/30620679},
doi = {10.2214/AJR.18.20180},
issn = {1546-3141},
year = {2019},
date = {2019-03-01},
urldate = {2019-03-01},
journal = {AJR. American journal of roentgenology},
volume = {212},
number = {3},
pages = {596-601},
abstract = {The objective of our study was to test for the possibility that published malignancy risks for side-branch intraductal papillary mucinous neoplasms (IPMNs) are overestimates, likely due to verification bias. We tested for possible verification bias using simulation modeling techniques. First, in age-defined hypothetical cohorts of 10 million persons, we projected the frequency of pancreatic ductal adenocarcinoma (PDAC) arising from side-branch IPMNs over 5 years using published estimates of their prevalence (4.4%) and rate of malignant transformation (1.9%). Second, we projected the total number of PDAC cases in corresponding cohorts over the same time horizon using national cancer registry data. For each cohort, we determined whether the percentage of all PDAC cases that arose from side-branch IPMNs (i.e., side-branch IPMN-associated PDAC cases) was clinically plausible using an upper limit of 10% to define plausibility, as estimated from the literature. Model assumptions and parameter uncertainty were evaluated in sensitivity analysis. Across all cohorts, percentages of side-branch IPMN-associated PDACs greatly exceeded 10%. In the base case (mean age = 55.7 years), 80% of PDAC cases arose from side-branch IPMNs (7877/9786). In the oldest cohort evaluated (mean age = 75 years), this estimate was 76% (14,227/18,714). In a secondary analysis, we found that if an upper limit threshold of 10% for side-branch IPMN-associated PDAC was imposed, the model-predicted rate of malignancy for side-branch IPMNs would be less than 0.24% over a 5-year time horizon, substantially lower than most literature-based estimates. Our results suggest that reported malignancy risks associated with side-branch IPMNs are likely to be overestimates and imply the presence of verification bias.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Chen, Qiushi; Larochelle, Marc R.; Weaver, Davis; Lietz, Anna; Mueller, Peter P.; Mercaldo, Sarah Fletcher; Wakeman, Sarah E.; Freedberg, Kenneth A.; Raphel, Tiana; Knudsen, Amy; Pandharipande, Pari; Chhatwal, Jagpreet
Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States Journal Article
In: JAMA Network Open, vol. 2, no. 2, pp. e187621-e187621, 2019, ISSN: 2574-3805, ().
@article{10.1001/jamanetworkopen.2018.7621,
title = {Prevention of Prescription Opioid Misuse and Projected Overdose Deaths in the United States},
author = {Qiushi Chen and Marc R. Larochelle and Davis Weaver and Anna Lietz and Peter P. Mueller and Sarah Fletcher Mercaldo and Sarah E. Wakeman and Kenneth A. Freedberg and Tiana Raphel and Amy Knudsen and Pari Pandharipande and Jagpreet Chhatwal},
url = {https://dx.doi.org/10.1001/jamanetworkopen.2018.7621},
doi = {10.1001/jamanetworkopen.2018.7621},
issn = {2574-3805},
year = {2019},
date = {2019-02-01},
journal = {JAMA Network Open},
volume = {2},
number = {2},
pages = {e187621-e187621},
abstract = {Deaths due to opioid overdose have tripled in the last decade. Efforts to curb this trend have focused on restricting the prescription opioid supply; however, the near-term effects of such efforts are unknown.To project effects of interventions to lower prescription opioid misuse on opioid overdose deaths from 2016 to 2025.This system dynamics (mathematical) model of the US opioid epidemic projected outcomes of simulated individuals who engage in nonmedical prescription or illicit opioid use from 2016 to 2025. The analysis was performed in 2018 by retrospectively calibrating the model from 2002 to 2015 data from the National Survey on Drug Use and Health and the Centers for Disease Control and Prevention.Comparison of interventions that would lower the incidence of prescription opioid misuse from 2016 to 2025 based on historical trends (a 7.5% reduction per year) and 50% faster than historical trends (an 11.3% reduction per year), vs a circumstance in which the incidence of misuse remained constant after 2015.Opioid overdose deaths from prescription and illicit opioids from 2016 to 2025 under each intervention.Under the status quo, the annual number of opioid overdose deaths is projected to increase from 33 100 in 2015 to 81 700 (95% uncertainty interval [UI], 63 600-101 700) in 2025 (a 147% increase from 2015). From 2016 to 2025, 700 400 (95% UI, 590 200-817 100) individuals in the United States are projected to die from opioid overdose, with 80% of the deaths attributable to illicit opioids. The number of individuals using illicit opioids is projected to increase by 61%\textemdashfrom 0.93 million (95% UI, 0.83-1.03 million) in 2015 to 1.50 million (95% UI, 0.98-2.22 million) by 2025. Across all interventions tested, further lowering the incidence of prescription opioid misuse from 2015 levels is projected to decrease overdose deaths by only 3.0% to 5.3%.This study’s findings suggest that interventions targeting prescription opioid misuse such as prescription monitoring programs may have a modest effect, at best, on the number of opioid overdose deaths in the near future. Additional policy interventions are urgently needed to change the course of the epidemic.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Lietz, Anna; Weaver, Davis; Melamed, Alexander; Rauh-Hain, Jose Alejandro; Wright, Jason D; Wright, Alexi; Knudsen, Amy; Pandharipande, Pari
Potential survival benefits from optimized chemotherapy implementation in advanced ovarian cancer: Projections from a microsimulation model. Journal Article
In: PloS one, vol. 14, pp. e0222828, 2019, ISSN: 1932-6203, ().
@article{Lietz2019,
title = {Potential survival benefits from optimized chemotherapy implementation in advanced ovarian cancer: Projections from a microsimulation model.},
author = {Anna Lietz and Davis Weaver and Alexander Melamed and Jose Alejandro Rauh-Hain and Jason D Wright and Alexi Wright and Amy Knudsen and Pari Pandharipande},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31539415},
doi = {10.1371/journal.pone.0222828},
issn = {1932-6203},
year = {2019},
date = {2019-01-01},
journal = {PloS one},
volume = {14},
pages = {e0222828},
abstract = {Ovarian cancer is often diagnosed in advanced stages, when survival is poor. Treatment advances have been made, but are inconsistently implemented. Our purpose was to project the maximum life expectancy gains that could be achieved in women with stage IIIC epithelial ovarian cancer if the implementation of available chemotherapy regimens could be optimized. We used a microsimulation model to estimate life expectancy benefits associated with "optimized" implementation of four post-operative chemotherapy options: standard intravenous chemotherapy; intraperitoneal + intravenous chemotherapy; bevacizumab + intravenous chemotherapy; and hyperthermic intraperitoneal chemotherapy + intravenous chemotherapy. Optimized implementation was defined as follows. Patients triaged to primary cytoreductive surgery received intraperitoneal + intravenous chemotherapy if optimally or completely cytoreduced, and bevacizumab + intravenous chemotherapy if suboptimally cytoreduced. Patients triaged to neoadjuvant chemotherapy received hyperthermic intraperitoneal chemotherapy at interval cytoreductive surgery if optimally or completely cytoreduced, and standard IV chemotherapy if suboptimally cytoreduced. Life expectancy associated with optimized implementation was compared with that of current utilization practices, estimated using published literature and the National Cancer Database. Effects of model uncertainty were evaluated in sensitivity analyses. Life expectancy associated with optimized implementation vs. current practice was 76.7 vs. 64.5 months (life expectancy gain = 12.2 months). Providing intraperitoneal + intravenous chemotherapy to all eligible patients was the largest driver of life expectancy gains, due to both the potential benefit conferred by intraperitoneal + intravenous chemotherapy and the proportion of eligible women who do not receive intraperitoneal + intravenous chemotherapy in current practice. Population-level life expectancy in stage IIIC epithelial ovarian cancer could be substantially improved through greater uptake of available chemotherapy regimens.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Naber, Steffie K; Knudsen, Amy; Zauber, Ann G; Rutter, Carolyn M; Fischer, Sara E; Pabiniak, Chester J; Soto, Brittany; Kuntz, Karen M; Lansdorp-Vogelaar, Iris
Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. Journal Article
In: PloS one, vol. 14, pp. e0220234, 2019, ISSN: 1932-6203, ().
@article{Naber2019,
title = {Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries.},
author = {Steffie K Naber and Amy Knudsen and Ann G Zauber and Carolyn M Rutter and Sara E Fischer and Chester J Pabiniak and Brittany Soto and Karen M Kuntz and Iris Lansdorp-Vogelaar},
url = {https://www.ncbi.nlm.nih.gov/pubmed/31483796},
doi = {10.1371/journal.pone.0220234},
issn = {1932-6203},
year = {2019},
date = {2019-01-01},
journal = {PloS one},
volume = {14},
pages = {e0220234},
abstract = {In 2014, the Centers for Medicare and Medicaid Services (CMS) began covering a multitarget stool DNA (mtSDNA) test for colorectal cancer (CRC) screening of Medicare beneficiaries. In this study, we evaluated whether mtSDNA testing is a cost-effective alternative to other CRC screening strategies reimbursed by CMS, and if not, under what conditions it could be. We use three independently-developed microsimulation models to simulate a cohort of previously unscreened US 65-year-olds who are screened with triennial mtSDNA testing, or one of six other reimbursed screening strategies. Main outcome measures are discounted life-years gained (LYG) and lifetime costs (CMS perspective), threshold reimbursement rates, and threshold adherence rates. Outcomes are expressed as the median and range across models. Compared to no screening, triennial mtSDNA screening resulted in 82 (range: 79-88) LYG per 1,000 simulated individuals. This was more than for five-yearly sigmoidoscopy (80 (range: 71-89) LYG), but fewer than for every other simulated strategy. At its 2017 reimbursement rate of $512, mtSDNA was the most costly strategy, and even if adherence were 30% higher than with other strategies, it would not be a cost-effective alternative. At a substantially reduced reimbursement rate ($6-18), two models found that triennial mtSDNA testing was an efficient and potentially cost-effective screening option. Compared to no screening, triennial mtSDNA screening reduces CRC incidence and mortality at acceptable costs. However, compared to nearly all other CRC screening strategies reimbursed by CMS it is less effective and considerably more costly, making it an inefficient screening option.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2018
Neal, Chrishanae; Weaver, Davis; Raphel, Tiana; Lietz, Anna; Flores, Efren J; Percac-Lima, Sanja; Knudsen, Amy; Pandharipande, Pari
Patient Navigation to Improve Cancer Screening in Underserved Populations: Reported Experiences, Opportunities, and Challenges Journal Article
In: Journal of the American College of Radiology : JACR, vol. 15, no. 11, pp. 1565-1572, 2018, ISSN: 1558-349X, ().
@article{Neal2018,
title = {Patient Navigation to Improve Cancer Screening in Underserved Populations: Reported Experiences, Opportunities, and Challenges},
author = {Chrishanae Neal and Davis Weaver and Tiana Raphel and Anna Lietz and Efren J Flores and Sanja Percac-Lima and Amy Knudsen and Pari Pandharipande},
url = {http://www.ncbi.nlm.nih.gov/pubmed/29685346},
doi = {10.1016/j.jacr.2018.03.001},
issn = {1558-349X},
year = {2018},
date = {2018-11-01},
journal = {Journal of the American College of Radiology : JACR},
volume = {15},
number = {11},
pages = {1565-1572},
abstract = {Our goal is to define patient navigation for an imaging audience, present a focused selection of published experiences with navigation programs for breast and colorectal cancer screening, and expose principal barriers to the success of such programs. Despite numerous advances in the early detection of cancers, many patients still present with advanced disease. A disproportionate number are low-income minority patients who experience worse health outcomes than their white or more financially stable counterparts. Patient navigation, which aims to assist the medically underserved by overcoming specific barriers to care, may represent one solution to narrowing disparities. Related research suggests that in general, patient navigation programs that have addressed breast or colorectal cancer screening have been successful in improving screening rates and timeliness of follow-up care. However, although beneficial, navigation is expensive and may present an unmanageable financial burden for many health care centers. To overcome this challenge, navigation efforts will likely need to target those patients that are most likely to benefit. Further research to identify such patients will be critically important for improving the sustainability of navigation programs, and, in turn, for realizing the benefits of such programs in reducing cancer disparities.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Meester, Reinier G S; Peterse, Elisabeth F P; Knudsen, Amy; Weerdt, Anne C; Chen, Jennifer C; Lietz, Anna; Dwyer, Andrea; Ahnen, Dennis J; Siegel, Rebecca L; Smith, Robert A; Zauber, Ann G; Lansdorp-Vogelaar, Iris
In: Cancer, vol. 124, no. 15, pp. 2974-2985, 2018, ISSN: 1097-0142, ().
@article{Meester2018,
title = {Optimizing colorectal cancer screening by race and sex: Microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline.},
author = {Reinier G S Meester and Elisabeth F P Peterse and Amy Knudsen and Anne C Weerdt and Jennifer C Chen and Anna Lietz and Andrea Dwyer and Dennis J Ahnen and Rebecca L Siegel and Robert A Smith and Ann G Zauber and Iris Lansdorp-Vogelaar},
url = {http://www.ncbi.nlm.nih.gov/pubmed/29846942},
doi = {10.1002/cncr.31542},
issn = {1097-0142},
year = {2018},
date = {2018-07-01},
urldate = {2018-07-01},
journal = {Cancer},
volume = {124},
number = {15},
pages = {2974-2985},
abstract = {Colorectal cancer (CRC) risk varies by race and sex. This study, 1 of 2 microsimulation analyses to inform the 2018 American Cancer Society CRC screening guideline, explored the influence of race and sex on optimal CRC screening strategies. Two Cancer Intervention and Surveillance Modeling Network microsimulation models, informed by US incidence data, were used to evaluate a variety of screening methods, ages to start and stop, and intervals for 4 demographic subgroups (black and white males and females) under 2 scenarios for the projected lifetime CRC risk for 40-year-olds: 1) assuming that risk had remained stable since the early screening era and 2) assuming that risk had increased proportionally to observed incidence trends under the age of 40 years. Model-based screening recommendations were based on the predicted level of benefit (life-years gained) and burden (required number of colonoscopies), the incremental burden-to-benefit ratio, and the relative efficiency in comparison with strategies with similar burdens. When lifetime CRC risk was assumed to be stable over time, the models differed in the recommended age to start screening for whites (45 vs 50 years) but consistently recommended screening from the age of 45 years for blacks. When CRC risk was assumed to be increased, the models recommended starting at the age of 45 years, regardless of race and sex. Strategies recommended under both scenarios included colonoscopy every 10 or 15 years, annual fecal immunochemical testing, and computed tomographic colonography every 5 years through the age of 75 years. Microsimulation modeling suggests that CRC screening should be considered from the age of 45 years for blacks and for whites if the lifetime risk has increased proportionally to the incidence for younger adults. Cancer 2018. © 2018 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Weaver, Davis; Raphel, Tiana; Melamed, Alexander; Rauh-Hain, Jose Alejandro; Schorge, John O; Knudsen, Amy; Pandharipande, Pari
Modeling treatment outcomes for patients with advanced ovarian cancer: Projected benefits of a test to optimize treatment selection. Journal Article
In: Gynecologic oncology, vol. 149, no. 2, pp. 256-262, 2018, ISSN: 1095-6859, ().
@article{Weaver2018,
title = {Modeling treatment outcomes for patients with advanced ovarian cancer: Projected benefits of a test to optimize treatment selection.},
author = {Davis Weaver and Tiana Raphel and Alexander Melamed and Jose Alejandro Rauh-Hain and John O Schorge and Amy Knudsen and Pari Pandharipande},
url = {http://www.ncbi.nlm.nih.gov/pubmed/29486993},
doi = {10.1016/j.ygyno.2018.02.007},
issn = {1095-6859},
year = {2018},
date = {2018-05-01},
journal = {Gynecologic oncology},
volume = {149},
number = {2},
pages = {256-262},
abstract = {For patients with advanced stage epithelial ovarian cancer (EOC), substantial emphasis has been placed on diagnostic tests that can discern which of two treatment options - primary cytoreductive surgery (PCS) or neoadjuvant chemotherapy followed by interval cytoreductive surgery (NACT+ICS) - optimizes patient-level outcomes. Our goal was to project potential life expectancy (LE) gains that could be achieved by use of such a test. We developed a microsimulation model to project LE for patients with stage IIIC EOC. We compared: a "standard-of-care" strategy, in which patients were triaged to PCS vs. NACT+ICS based on current clinical practice; and a "test" strategy, in which patients were triaged based on results of a hypothetical test. We identified those test performance characteristics for which the test strategy outperformed the standard-of-care strategy, from a LE standpoint. Effects of parameter uncertainty were evaluated in sensitivity analysis. Even with a perfect test, the LE gain was modest (LE with test vs. standard-of-care strategy=67.6 vs. 66.4months; LE gain=1.2months). In order to outperform the standard-of-care, the test had to have a high probability of correctly identifying "resectable" patients at PCS (i.e. those for whom complete or optimal cytoreduction would be possible); this test property was more important than correct triage of unresectable patients to NACT+ICS. Results were sensitive to the proportion of patients whose underlying disease was resectable at PCS. Diagnostic tests that are designed to triage patients with advanced stage EOC will likely have only a modest effect on LE.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Raphel, Tiana; Weaver, Davis; Berland, Lincoln L; Herts, Brian R; Megibow, Alec J; Knudsen, Amy; Pandharipande, Pari
Imaging Follow-up of Low-Risk Incidental Pancreas and Kidney Findings: Effects of Patient Age and Comorbidity on Projected Life Expectancy. Journal Article
In: Radiology, vol. 287, no. 2, pp. 504-514, 2018, ISSN: 1527-1315, ().
@article{Raphel2018,
title = {Imaging Follow-up of Low-Risk Incidental Pancreas and Kidney Findings: Effects of Patient Age and Comorbidity on Projected Life Expectancy.},
author = {Tiana Raphel and Davis Weaver and Lincoln L Berland and Brian R Herts and Alec J Megibow and Amy Knudsen and Pari Pandharipande},
url = {https://www.ncbi.nlm.nih.gov/pubmed/29401040},
doi = {10.1148/radiol.2018171701},
issn = {1527-1315},
year = {2018},
date = {2018-05-01},
urldate = {2018-05-01},
journal = {Radiology},
volume = {287},
number = {2},
pages = {504-514},
abstract = {Purpose To determine the effects of patient age and comorbidity level on life expectancy (LE) benefits associated with imaging follow-up of Bosniak IIF renal cysts and pancreatic side-branch (SB) intraductal papillary mucinous neoplasms (IPMNs). Materials and Methods A decision-analytic Markov model to evaluate LE benefits was developed. Hypothetical cohorts with varied age (60-80 years) and comorbidities (none, mild, moderate, or severe) were evaluated. For each finding, LE projections from two strategies were compared: imaging follow-up and no imaging follow-up. Under follow-up, it was assumed that cancers associated with the incidental finding were successfully treated before they spread. For patients without follow-up, mortality risks from Bosniak IIF cysts (renal cell carcinoma) and SBIPMNs (pancreatic ductal adenocarcinoma) were incorporated. Model assumptions and parameter uncertainty were evaluated in sensitivity analysis. Results In the youngest, healthiest cohorts (age, 60 years; no comorbidities), projected LE benefits from follow-up were as follows: Bosniak IIF cyst, 6.5 months (women) and 5.8 months (men); SBIPMN, 6.4 months (women) and 5.3 months (men). Follow-up of Bosniak IIF cysts in 60-year-old women with severe comorbidities yielded a LE benefit of 3.9 months; in 80-year-old women with no comorbidities, the benefit was 2.8 months, and with severe comorbidities the benefit was 1.5 months. Similar trends were observed in men and for SBIPMN. Results were sensitive to the performance of follow-up for cancer detection; malignancy risks; and stage at presentation of malignant, unfollowed Bosniak IIF cysts. Conclusion With progression of age and comorbidity level, follow-up of low-risk incidental findings yields increasingly limited benefits for patients. © RSNA, 2018 Online supplemental material is available for this article.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2016
Rutter, Carolyn M.; Knudsen, Amy; Marsh, Tracey L.; Doria-Rose, V Paul; Johnson, Eric; Pabiniak, Chester; Kuntz, Karen M.; Ballegooijen, Marjolein; Zauber, Ann G.; Lansdorp-Vogelaar, Iris
Validation of Models Used to Inform Colorectal Cancer Screening Guidelines: Accuracy and Implications Journal Article
In: Med Decis Making, vol. 36, no. 5, pp. 604-14, 2016, ().
@article{Rutter2016,
title = {Validation of Models Used to Inform Colorectal Cancer Screening Guidelines: Accuracy and Implications},
author = {Carolyn M. Rutter and Amy Knudsen and Tracey L. Marsh and V Paul Doria-Rose and Eric Johnson and Chester Pabiniak and Karen M. Kuntz and Marjolein Ballegooijen and Ann G. Zauber and Iris Lansdorp-Vogelaar},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26746432},
doi = {10.1177/0272989X15622642},
year = {2016},
date = {2016-07-01},
journal = {Med Decis Making},
volume = {36},
number = {5},
pages = {604-14},
institution = {Intervention Research Branch, Bethesda, MD, USA (VPD)Group Health Research Institute, Seattle, WA, USA (EJ, CP)Department of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA (KMK)Department of Public He},
abstract = {Microsimulation models synthesize evidence about disease processes and interventions, providing a method for predicting long-term benefits and harms of prevention, screening, and treatment strategies. Because models often require assumptions about unobservable processes, assessing a model's predictive accuracy is important.We validated 3 colorectal cancer (CRC) microsimulation models against outcomes from the United Kingdom Flexible Sigmoidoscopy Screening (UKFSS) Trial, a randomized controlled trial that examined the effectiveness of one-time flexible sigmoidoscopy screening to reduce CRC mortality. The models incorporate different assumptions about the time from adenoma initiation to development of preclinical and symptomatic CRC. Analyses compare model predictions to study estimates across a range of outcomes to provide insight into the accuracy of model assumptions.All 3 models accurately predicted the relative reduction in CRC mortality 10 years after screening (predicted hazard ratios, with 95% percentile intervals: 0.56 [0.44, 0.71], 0.63 [0.51, 0.75], 0.68 [0.53, 0.83]; estimated with 95% confidence interval: 0.56 [0.45, 0.69]). Two models with longer average preclinical duration accurately predicted the relative reduction in 10-year CRC incidence. Two models with longer mean sojourn time accurately predicted the number of screen-detected cancers. All 3 models predicted too many proximal adenomas among patients referred to colonoscopy.Model accuracy can only be established through external validation. Analyses such as these are therefore essential for any decision model. Results supported the assumptions that the average time from adenoma initiation to development of preclinical cancer is long (up to 25 years), and mean sojourn time is close to 4 years, suggesting the window for early detection and intervention by screening is relatively long. Variation in dwell time remains uncertain and could have important clinical and policy implications.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Knudsen, Amy; Zauber, Ann G.; Rutter, Carolyn M.; Naber, Steffie K.; Doria-Rose, V Paul; Pabiniak, Chester; Johanson, Colden; Fischer, Sara E.; Lansdorp-Vogelaar, Iris; Kuntz, Karen M.
Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force Journal Article
In: JAMA, vol. 315, no. 23, pp. 2595–2609, 2016, ().
@article{Knudsen2016,
title = {Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force},
author = {Amy Knudsen and Ann G. Zauber and Carolyn M. Rutter and Steffie K. Naber and V Paul Doria-Rose and Chester Pabiniak and Colden Johanson and Sara E. Fischer and Iris Lansdorp-Vogelaar and Karen M. Kuntz},
url = {http://www.ncbi.nlm.nih.gov/pubmed/27305518},
doi = {10.1001/jama.2016.6828},
year = {2016},
date = {2016-06-01},
urldate = {2016-06-01},
journal = {JAMA},
volume = {315},
number = {23},
pages = {2595--2609},
institution = {Department of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis.},
abstract = {The US Preventive Services Task Force (USPSTF) is updating its 2008 colorectal cancer (CRC) screening recommendations.To inform the USPSTF by modeling the benefits, burden, and harms of CRC screening strategies; estimating the optimal ages to begin and end screening; and identifying a set of model-recommendable strategies that provide similar life-years gained (LYG) and a comparable balance between LYG and screening burden.Comparative modeling with 3 microsimulation models of a hypothetical cohort of previously unscreened US 40-year-olds with no prior CRC diagnosis.Screening with sensitive guaiac-based fecal occult blood testing, fecal immunochemical testing (FIT), multitarget stool DNA testing, flexible sigmoidoscopy with or without stool testing, computed tomographic colonography (CTC), or colonoscopy starting at age 45, 50, or 55 years and ending at age 75, 80, or 85 years. Screening intervals varied by modality. Full adherence for all strategies was assumed.Life-years gained compared with no screening (benefit), lifetime number of colonoscopies required (burden), lifetime number of colonoscopy complications (harms), and ratios of incremental burden and benefit (efficiency ratios) per 1000 40-year-olds.The screening strategies provided LYG in the range of 152 to 313 per 1000 40-year-olds. Lifetime colonoscopy burden per 1000 persons ranged from fewer than 900 (FIT every 3 years from ages 55-75 years) to more than 7500 (colonoscopy screening every 5 years from ages 45-85 years). Harm from screening was at most 23 complications per 1000 persons screened. Strategies with screening beginning at age 50 years generally provided more LYG as well as more additional LYG per additional colonoscopy than strategies with screening beginning at age 55 years. There were limited empirical data to support a start age of 45 years. For persons adequately screened up to age 75 years, additional screening yielded small increases in LYG relative to the increase in colonoscopy burden. With screening from ages 50 to 75 years, 4 strategies yielded a comparable balance of screening burden and similar LYG (median LYG per 1000 across the models): colonoscopy every 10 years (270 LYG); sigmoidoscopy every 10 years with annual FIT (256 LYG); CTC every 5 years (248 LYG); and annual FIT (244 LYG).In this microsimulation modeling study of a previously unscreened population undergoing CRC screening that assumed 100% adherence, the strategies of colonoscopy every 10 years, annual FIT, sigmoidoscopy every 10 years with annual FIT, and CTC every 5 years performed from ages 50 through 75 years provided similar LYG and a comparable balance of benefit and screening burden.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2015
Goede, Simon L.; Kuntz, Karen M.; Ballegooijen, Marjolein; Knudsen, Amy; Lansdorp-Vogelaar, Iris; Tangka, Florence K.; Howard, David H.; Chin, Joseph; Zauber, Ann G.; Seeff, Laura C.
Cost-Savings to Medicare From Pre-Medicare Colorectal Cancer Screening Journal Article
In: Med Care, vol. 53, no. 7, pp. 630–638, 2015, ().
@article{Goede2015,
title = {Cost-Savings to Medicare From Pre-Medicare Colorectal Cancer Screening},
author = {Simon L. Goede and Karen M. Kuntz and Marjolein Ballegooijen and Amy Knudsen and Iris Lansdorp-Vogelaar and Florence K. Tangka and David H. Howard and Joseph Chin and Ann G. Zauber and Laura C. Seeff},
url = {http://www.ncbi.nlm.nih.gov/pubmed/26067885},
doi = {10.1097/MLR.0000000000000380},
year = {2015},
date = {2015-07-01},
journal = {Med Care},
volume = {53},
number = {7},
pages = {630--638},
institution = {*Department of Public Health, Erasmus University Medical Center, Rotterdam, The Netherlands †Division of Health Policy and Management, University of Minnesota, Minneapolis, MN ‡Department of Radiology, Institute for Technology Assessment, Massachusetts Ge},
abstract = {Many individuals have not received recommended colorectal cancer (CRC) screening before they become Medicare eligible at the age of 65. We aimed to estimate the long-term implications of increased CRC screening in the pre-Medicare population (50-64 y) on costs in the pre-Medicare and Medicare populations (65+ y).We used 2 independently developed microsimulation models [Microsimulation Screening Analysis Colon (MISCAN) and Simulation Model of CRC (SimCRC)] to project CRC screening and treatment costs under 2 scenarios, starting in 2010: "current trends" (60% of the population up-to-date with screening recommendations) and "enhanced participation" (70% up-to-date). The population was scaled to the projected US population for each year between 2010 and 2060. Costs per year were derived by age group (50-64 and 65+ y).By 2060, the discounted cumulative total costs in the pre-Medicare population were $35.7 and $28.1 billion higher with enhanced screening participation, than in the current trends scenario ($252.1 billion with MISCAN and $239.5 billion with SimCRC, respectively). Because of CRC treatment savings with enhanced participation, cumulative costs in the Medicare population were $18.3 and $32.7 billion lower (current trends: $423.5 billion with MISCAN and $372.8 billion with SimCRC). Over the 50-year time horizon an estimated 60% (MISCAN) and 89% (SimCRC) of the increased screening costs could be offset by savings in Medicare CRC treatment costs.Increased CRC screening participation in the pre-Medicare population could reduce CRC incidence and mortality, whereas the additional screening costs can be largely offset by long-term Medicare treatment savings.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Steen, Alex; Knudsen, Amy; Hees, Frank; Walter, Gailya P.; Berger, Franklin G.; Daguise, Virginie G.; Kuntz, Karen M.; Zauber, Ann G.; Ballegooijen, Marjolein; Lansdorp-Vogelaar, Iris
Optimal colorectal cancer screening in states' low-income, uninsured populations-the case of South Carolina Journal Article
In: Health Serv Res, vol. 50, no. 3, pp. 768–789, 2015, ().
@article{vanderSteen2015,
title = {Optimal colorectal cancer screening in states' low-income, uninsured populations-the case of South Carolina},
author = {Alex Steen and Amy Knudsen and Frank Hees and Gailya P. Walter and Franklin G. Berger and Virginie G. Daguise and Karen M. Kuntz and Ann G. Zauber and Marjolein Ballegooijen and Iris Lansdorp-Vogelaar},
url = {http://www.ncbi.nlm.nih.gov/pubmed/25324198},
doi = {10.1111/1475-6773.12246},
year = {2015},
date = {2015-06-01},
urldate = {2015-06-01},
journal = {Health Serv Res},
volume = {50},
number = {3},
pages = {768--789},
institution = {Department of Public Health, Erasmus MC, Rotterdam, The Netherlands.},
abstract = {To determine whether, given a limited budget, a state's low-income uninsured population would have greater benefit from a colorectal cancer (CRC) screening program using colonoscopy or fecal immunochemical testing (FIT).South Carolina's low-income, uninsured population.Comparative effectiveness analysis using microsimulation modeling to estimate the number of individuals screened, CRC cases prevented, CRC deaths prevented, and life-years gained from a screening program using colonoscopy versus a program using annual FIT in South Carolina's low-income, uninsured population. This analysis assumed an annual budget of $1 million and a budget availability of 2 years as a base case.The annual FIT screening program resulted in nearly eight times more individuals being screened, and more important, approximately four times as many CRC deaths prevented and life-years gained than the colonoscopy screening program. Our results were robust for assumptions concerning economic perspective and the target population, and they may therefore be generalized to other states and populations.A FIT screening program will prevent more CRC deaths than a colonoscopy-based program when a state's budget for CRC screening supports screening of only a fraction of the target population.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2013
Rutter, Carolyn M.; Johnson, Eric A.; Feuer, Eric J.; Knudsen, Amy; Kuntz, Karen M.; Schrag, Deborah
Secular Trends in Colon and Rectal Cancer Relative Survival Journal Article
In: J Natl Cancer Inst, vol. 105, no. 23, pp. 1806-13, 2013, ().
@article{Rutter2013,
title = {Secular Trends in Colon and Rectal Cancer Relative Survival},
author = {Carolyn M. Rutter and Eric A. Johnson and Eric J. Feuer and Amy Knudsen and Karen M. Kuntz and Deborah Schrag},
url = {http://www.ncbi.nlm.nih.gov/pubmed/24174654},
doi = {10.1093/jnci/djt299},
year = {2013},
date = {2013-12-01},
urldate = {2013-12-01},
journal = {J Natl Cancer Inst},
volume = {105},
number = {23},
pages = {1806-13},
institution = {Affiliations of authors: Group Health Research Institute, Seattle, WA (CMR, EAJ); Division of Cancer Control and Population Sciences National Cancer Institute, Bethesda, MD (EJF); Institute for Technology Assessment, Department of Radiology, Massach},
abstract = {Treatment options for colorectal cancer (CRC) have improved substantially
over the past 25 years. Measuring the impact of these improvements
on survival outcomes is challenging, however, against the background
of overall survival gains from advancements in the prevention, screening,
and treatment of other conditions. Relative survival is a metric
that accounts for these concurrent changes, allowing assessment of
changes in CRC survival. We describe stage- and location-specific
trends in relative survival after CRC diagnosis.We analyzed survival
outcomes for 233965 people in the Surveillance Epidemiology and End
Results (SEER) program who were diagnosed with CRC between January
1, 1975, and December 31, 2003. All models were adjusted for sex,
race (black vs white), age at diagnosis, time since diagnosis, and
diagnosis year. We estimated the proportional difference in survival
for CRC patients compared with overall survival for age-, sex-, race-,
and period-matched controls to account for concurrent changes in
overall survival using two-sided Wald tests.We found statistically
significant reductions in excess hazard of mortality from CRC in
2003 relative to 1975, with excess hazard ratios ranging from 0.75
(stage IV colon cancer; P},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
over the past 25 years. Measuring the impact of these improvements
on survival outcomes is challenging, however, against the background
of overall survival gains from advancements in the prevention, screening,
and treatment of other conditions. Relative survival is a metric
that accounts for these concurrent changes, allowing assessment of
changes in CRC survival. We describe stage- and location-specific
trends in relative survival after CRC diagnosis.We analyzed survival
outcomes for 233965 people in the Surveillance Epidemiology and End
Results (SEER) program who were diagnosed with CRC between January
1, 1975, and December 31, 2003. All models were adjusted for sex,
race (black vs white), age at diagnosis, time since diagnosis, and
diagnosis year. We estimated the proportional difference in survival
for CRC patients compared with overall survival for age-, sex-, race-,
and period-matched controls to account for concurrent changes in
overall survival using two-sided Wald tests.We found statistically
significant reductions in excess hazard of mortality from CRC in
2003 relative to 1975, with excess hazard ratios ranging from 0.75
(stage IV colon cancer; P
2012
Knudsen, Amy; Hur, Chin; Gazelle, G. Scott; Schrag, Deborah; McFarland, Elizabeth G.; Kuntz, Karen M.
Rescreening of persons with a negative colonoscopy result: results from a microsimulation model Journal Article
In: Ann Intern Med, vol. 157, no. 9, pp. 611–620, 2012, ().
@article{Knudsen2012,
title = {Rescreening of persons with a negative colonoscopy result: results from a microsimulation model},
author = {Amy Knudsen and Chin Hur and G. Scott Gazelle and Deborah Schrag and Elizabeth G. McFarland and Karen M. Kuntz},
url = {http://www.ncbi.nlm.nih.gov/pubmed/23128861},
doi = {10.7326/0003-4819-157-9-201211060-00005},
year = {2012},
date = {2012-11-01},
journal = {Ann Intern Med},
volume = {157},
number = {9},
pages = {611--620},
abstract = {Persons with a negative result on screening colonoscopy are recommended to repeat the procedure in 10 years.To assess the effectiveness and costs of colonoscopy versus other rescreening strategies after an initial negative colonoscopy result.Microsimulation model.Literature and data from the Surveillance, Epidemiology, and End Results program.Persons aged 50 years who had no adenomas or cancer detected on screening colonoscopy.Lifetime.Societal.No further screening or rescreening starting at age 60 years with colonoscopy every 10 years, annual highly sensitive guaiac fecal occult blood testing (HSFOBT), annual fecal immunochemical testing (FIT), or computed tomographic colonography (CTC) every 5 years.Lifetime cases of colorectal cancer, life expectancy, and lifetime costs per 1000 persons, assuming either perfect or imperfect adherence.Rescreening with any method substantially reduced the risk for colorectal cancer compared with no further screening (range, 7.7 to 12.6 lifetime cases per 1000 persons [perfect adherence] and 17.7 to 20.9 lifetime cases per 1000 persons [imperfect adherence] vs. 31.3 lifetime cases per 1000 persons with no further screening).
In both adherence scenarios, the differences in life-years across rescreening strategies were small (range, 30 893 to 30 902 life-years per 1000 persons [perfect adherence] vs. 30 865 to 30 869 life-years per 1000 persons [imperfect adherence]). Rescreening with HSFOBT, FIT, or CTC had fewer complications and was less costly than continuing colonoscopy.Results were sensitive to test-specific adherence rates.Data on adherence to rescreening were limited.Compared with the currently recommended strategy of continuing colonoscopy every 10 years after an initial negative examination, rescreening at age 60 years with annual HSFOBT, annual FIT, or CTC every 5 years provides approximately the same benefit in life-years with fewer complications at a lower cost. Therefore, it is reasonable to use other methods to rescreen persons with negative colonoscopy results.National Cancer Institute.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
In both adherence scenarios, the differences in life-years across rescreening strategies were small (range, 30 893 to 30 902 life-years per 1000 persons [perfect adherence] vs. 30 865 to 30 869 life-years per 1000 persons [imperfect adherence]). Rescreening with HSFOBT, FIT, or CTC had fewer complications and was less costly than continuing colonoscopy.Results were sensitive to test-specific adherence rates.Data on adherence to rescreening were limited.Compared with the currently recommended strategy of continuing colonoscopy every 10 years after an initial negative examination, rescreening at age 60 years with annual HSFOBT, annual FIT, or CTC every 5 years provides approximately the same benefit in life-years with fewer complications at a lower cost. Therefore, it is reasonable to use other methods to rescreen persons with negative colonoscopy results.National Cancer Institute.
2011
Vanness, D. J.; Knudsen, Amy; Lansdorp-Vogelaar, I.; Rutter, C. M.; Gareen, I. F.; Herman, B. A.; Kuntz, K. M.; Zauber, A. G.; Ballegooijen, M.; Feuer, E. J.; Chen, M. H.; Johnson, C. D.
In: Radiology, vol. 261, no. 2, pp. 487-98, 2011, ISSN: 1527-1315 (Electronic) 0033-8419, ().
@article{Vanness2011,
title = {Comparative economic evaluation of data from the ACRIN National CT
Colonography {T}rial with three cancer intervention and surveillance
modeling network microsimulations},
author = {D. J. Vanness and Amy Knudsen and I. Lansdorp-Vogelaar and C. M. Rutter and I. F. Gareen and B. A. Herman and K. M. Kuntz and A. G. Zauber and M. Ballegooijen and E. J. Feuer and M. H. Chen and C. D. Johnson},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21813740},
issn = {1527-1315 (Electronic) 0033-8419},
year = {2011},
date = {2011-11-01},
journal = {Radiology},
volume = {261},
number = {2},
pages = {487-98},
abstract = {PURPOSE: To estimate the cost-effectiveness of computed tomographic
(CT) colonography for colorectal cancer (CRC) screening in average-risk
asymptomatic subjects in the United States aged 50 years. MATERIALS
AND METHODS: Enrollees in the American College of Radiology Imaging
Network National CT Colonography Trial provided informed consent,
and approval was obtained from the institutional review board at
each site. CT colonography performance estimates from the trial were
incorporated into three Cancer Intervention and Surveillance Modeling
Network CRC microsimulations. Simulated survival and lifetime costs
for screening 50-year-old subjects in the United States with CT colonography
every 5 or 10 years were compared with those for guideline-concordant
screening with colonoscopy, flexible sigmoidoscopy plus either sensitive
unrehydrated fecal occult blood testing (FOBT) or fecal immunochemical
testing (FIT), and no screening. Perfect and reduced screening adherence
scenarios were considered. Incremental cost-effectiveness and net
health benefits were estimated from the U.S. health care sector perspective,
assuming a 3% discount rate. RESULTS: CT colonography at 5- and 10-year
screening intervals was more costly and less effective than FOBT
plus flexible sigmoidoscopy in all three models in both 100% and
50% adherence scenarios. Colonoscopy also was more costly and less
effective than FOBT plus flexible sigmoidoscopy, except in the CRC-SPIN
model assuming 100% adherence (incremental cost-effectiveness ratio:
$26,300 per life-year gained). CT colonography at 5- and 10-year
screening intervals and colonoscopy were net beneficial compared
with no screening in all model scenarios. The 5-year screening interval
was net beneficial over the 10-year interval except in the MISCAN
model when assuming 100% adherence and willingness to pay $50,000
per life-year gained. CONCLUSION: All three models predict CT colonography
to be more costly and less effective than non-CT colonographic screening
but net beneficial compared with no screening given model assumptions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
(CT) colonography for colorectal cancer (CRC) screening in average-risk
asymptomatic subjects in the United States aged 50 years. MATERIALS
AND METHODS: Enrollees in the American College of Radiology Imaging
Network National CT Colonography Trial provided informed consent,
and approval was obtained from the institutional review board at
each site. CT colonography performance estimates from the trial were
incorporated into three Cancer Intervention and Surveillance Modeling
Network CRC microsimulations. Simulated survival and lifetime costs
for screening 50-year-old subjects in the United States with CT colonography
every 5 or 10 years were compared with those for guideline-concordant
screening with colonoscopy, flexible sigmoidoscopy plus either sensitive
unrehydrated fecal occult blood testing (FOBT) or fecal immunochemical
testing (FIT), and no screening. Perfect and reduced screening adherence
scenarios were considered. Incremental cost-effectiveness and net
health benefits were estimated from the U.S. health care sector perspective,
assuming a 3% discount rate. RESULTS: CT colonography at 5- and 10-year
screening intervals was more costly and less effective than FOBT
plus flexible sigmoidoscopy in all three models in both 100% and
50% adherence scenarios. Colonoscopy also was more costly and less
effective than FOBT plus flexible sigmoidoscopy, except in the CRC-SPIN
model assuming 100% adherence (incremental cost-effectiveness ratio:
$26,300 per life-year gained). CT colonography at 5- and 10-year
screening intervals and colonoscopy were net beneficial compared
with no screening in all model scenarios. The 5-year screening interval
was net beneficial over the 10-year interval except in the MISCAN
model when assuming 100% adherence and willingness to pay $50,000
per life-year gained. CONCLUSION: All three models predict CT colonography
to be more costly and less effective than non-CT colonographic screening
but net beneficial compared with no screening given model assumptions.
Rutter, C. M.; Knudsen, Amy; Pandharipande, Pari
Computer disease simulation models: integrating evidence for health policy Journal Article
In: Acad Radiol, vol. 18, no. 9, pp. 1077-86, 2011, ISSN: 1878-4046 (Electronic) 1076-6332, ().
@article{Rutter2011,
title = {Computer disease simulation models: integrating evidence for health
policy},
author = {C. M. Rutter and Amy Knudsen and Pari Pandharipande},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21435924},
issn = {1878-4046 (Electronic) 1076-6332},
year = {2011},
date = {2011-09-01},
journal = {Acad Radiol},
volume = {18},
number = {9},
pages = {1077-86},
abstract = {Computer disease simulation models are increasingly being used to
evaluate and inform health care decisions across medical disciplines.
The aim of researchers who develop these models is to integrate and
synthesize short-term outcomes and results from multiple sources
to predict the long-term clinical outcomes and costs of different
health care strategies. Policy makers, in turn, can use the predictions
generated by disease models together with other evidence to make
decisions related to health care practices and resource utilization.
Models are particularly useful when the existing evidence does not
yield obvious answers or does not provide answers to the questions
of greatest interest, such as questions about the relative cost-effectiveness
of different practices. This review focuses on models used to inform
decisions about imaging technology, discussing the role of disease
models for health policy development and providing a foundation for
understanding the basic principles of disease modeling. This manuscript
draws from the collective computed tomographic colonography modeling
experience, reviewing 10 published investigations of the clinical
effectiveness and cost-effectiveness of computed tomographic colonography
relative to colonoscopy. The discussion focuses on implications of
different modeling assumptions and difficulties that may be encountered
when evaluating the quality of models. This underscores the importance
of forging stronger collaborations between researchers who develop
disease models and radiologists, to ensure that policy-level models
accurately represent the experience of everyday clinical practices.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
evaluate and inform health care decisions across medical disciplines.
The aim of researchers who develop these models is to integrate and
synthesize short-term outcomes and results from multiple sources
to predict the long-term clinical outcomes and costs of different
health care strategies. Policy makers, in turn, can use the predictions
generated by disease models together with other evidence to make
decisions related to health care practices and resource utilization.
Models are particularly useful when the existing evidence does not
yield obvious answers or does not provide answers to the questions
of greatest interest, such as questions about the relative cost-effectiveness
of different practices. This review focuses on models used to inform
decisions about imaging technology, discussing the role of disease
models for health policy development and providing a foundation for
understanding the basic principles of disease modeling. This manuscript
draws from the collective computed tomographic colonography modeling
experience, reviewing 10 published investigations of the clinical
effectiveness and cost-effectiveness of computed tomographic colonography
relative to colonoscopy. The discussion focuses on implications of
different modeling assumptions and difficulties that may be encountered
when evaluating the quality of models. This underscores the importance
of forging stronger collaborations between researchers who develop
disease models and radiologists, to ensure that policy-level models
accurately represent the experience of everyday clinical practices.
Lansdorp-Vogelaar, I.; Knudsen, Amy; Brenner, H.
Cost-effectiveness of colorectal cancer screening Journal Article
In: Epidemiol Rev, vol. 33, no. 1, pp. 88-100, 2011, ISSN: 1478-6729 (Electronic) 0193-936X, ().
@article{Lansdorp-Vogelaar2011,
title = {Cost-effectiveness of colorectal cancer screening},
author = {I. Lansdorp-Vogelaar and Amy Knudsen and H. Brenner},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21633092},
issn = {1478-6729 (Electronic) 0193-936X},
year = {2011},
date = {2011-07-01},
journal = {Epidemiol Rev},
volume = {33},
number = {1},
pages = {88-100},
abstract = {Colorectal cancer is an important public health problem. Several screening
methods have been shown to be effective in reducing colorectal cancer
mortality. The objective of this review was to assess the cost-effectiveness
of the different colorectal cancer screening methods and to determine
the preferred method from a cost-effectiveness point of view. Five
databases (MEDLINE, EMBASE, the Cost-Effectiveness Analysis Registry,
the British National Health Service Economic Evaluation Database,
and the lists of technology assessments of the Centers for Medicare
and Medicaid Services) were searched for cost-effectiveness analyses
published in English between January 1993 and December 2009. Fifty-five
publications relating to 32 unique cost-effectiveness models were
identified. All studies found that colorectal cancer screening was
cost-effective or even cost-saving compared with no screening. However,
the studies disagreed as to which screening method was most effective
or had the best incremental cost-effectiveness ratio for a given
willingness to pay per life-year gained. There was agreement among
studies that the newly developed screening tests of stool DNA testing,
computed tomographic colonography, and capsule endoscopy were not
yet cost-effective compared with the established screening options.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
methods have been shown to be effective in reducing colorectal cancer
mortality. The objective of this review was to assess the cost-effectiveness
of the different colorectal cancer screening methods and to determine
the preferred method from a cost-effectiveness point of view. Five
databases (MEDLINE, EMBASE, the Cost-Effectiveness Analysis Registry,
the British National Health Service Economic Evaluation Database,
and the lists of technology assessments of the Centers for Medicare
and Medicaid Services) were searched for cost-effectiveness analyses
published in English between January 1993 and December 2009. Fifty-five
publications relating to 32 unique cost-effectiveness models were
identified. All studies found that colorectal cancer screening was
cost-effective or even cost-saving compared with no screening. However,
the studies disagreed as to which screening method was most effective
or had the best incremental cost-effectiveness ratio for a given
willingness to pay per life-year gained. There was agreement among
studies that the newly developed screening tests of stool DNA testing,
computed tomographic colonography, and capsule endoscopy were not
yet cost-effective compared with the established screening options.
Haug, U.; Kuntz, K. M.; Knudsen, Amy; Hundt, S.; Brenner, H.
Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia Journal Article
In: Br J Cancer, vol. 104, no. 11, pp. 1779-85, 2011, ISSN: 1532-1827 (Electronic) 0007-0920, ().
@article{Haug2011,
title = {Sensitivity of immunochemical faecal occult blood testing for detecting
left- vs right-sided colorectal neoplasia},
author = {U. Haug and K. M. Kuntz and Amy Knudsen and S. Hundt and H. Brenner},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21559011},
issn = {1532-1827 (Electronic) 0007-0920},
year = {2011},
date = {2011-05-01},
journal = {Br J Cancer},
volume = {104},
number = {11},
pages = {1779-85},
abstract = {BACKGROUND: Faecal occult blood tests (FOBTs) are used for colorectal
cancer (CRC) screening. We aimed to assess the sensitivity of an
immunochemical FOBT for detecting advanced colorectal neoplasia in
the left vs the right colon and to explore reasons for potential
differences in site-specific test performance. METHODS: We prospectively
measured faecal occult blood levels by a quantitative immunochemical
FOBT (RIDASCREEN) in 2310 average-risk subjects undergoing screening
colonoscopy. We compared diagnostic performance for subjects with
left- vs right-sided advanced neoplasia, as well as patient characteristics
and adenoma characteristics that have been suggested to impact faecal
haemoglobin levels. RESULTS: Sensitivities for subjects with left-
vs right-sided advanced neoplasia were 33% (95% confidence interval (CI), 26-41%) and 20% (CI, 11-31%) (P=0.04) at a specificity of 95%
(overall sensitivity: 29%) and the areas under the receiver-operating
characteristics curve were 0.71 (CI, 0.69-0.72) and 0.60 (CI, 0.58-0.63),
respectively. Pedunculated shape was strikingly more common in participants
with left- vs right-sided advanced neoplasia (47% vs 14%). In logistic
regression analyses adjusted for site, pedunculated shape was statistically significantly associated with test sensitivity (P=0.04). CONCLUSIONS:
The immunochemical FOBT in our study was more sensitive for detecting
subjects with left- vs right-sided advanced colorectal neoplasia.
Our findings may stimulate further diagnostic research in the field
as well as modelling analyses to estimate the potential effect of
site-specific test performance on the effectiveness of annual or
biennial FOBT-based screening programmes, in particular with respect
to protection from right-sided CRC.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
cancer (CRC) screening. We aimed to assess the sensitivity of an
immunochemical FOBT for detecting advanced colorectal neoplasia in
the left vs the right colon and to explore reasons for potential
differences in site-specific test performance. METHODS: We prospectively
measured faecal occult blood levels by a quantitative immunochemical
FOBT (RIDASCREEN) in 2310 average-risk subjects undergoing screening
colonoscopy. We compared diagnostic performance for subjects with
left- vs right-sided advanced neoplasia, as well as patient characteristics
and adenoma characteristics that have been suggested to impact faecal
haemoglobin levels. RESULTS: Sensitivities for subjects with left-
vs right-sided advanced neoplasia were 33% (95% confidence interval (CI), 26-41%) and 20% (CI, 11-31%) (P=0.04) at a specificity of 95%
(overall sensitivity: 29%) and the areas under the receiver-operating
characteristics curve were 0.71 (CI, 0.69-0.72) and 0.60 (CI, 0.58-0.63),
respectively. Pedunculated shape was strikingly more common in participants
with left- vs right-sided advanced neoplasia (47% vs 14%). In logistic
regression analyses adjusted for site, pedunculated shape was statistically significantly associated with test sensitivity (P=0.04). CONCLUSIONS:
The immunochemical FOBT in our study was more sensitive for detecting
subjects with left- vs right-sided advanced colorectal neoplasia.
Our findings may stimulate further diagnostic research in the field
as well as modelling analyses to estimate the potential effect of
site-specific test performance on the effectiveness of annual or
biennial FOBT-based screening programmes, in particular with respect
to protection from right-sided CRC.
Gonzalez, A. Berrington; Kim, K. P.; Knudsen, Amy; Lansdorp-Vogelaar, I.; Rutter, C. M.; Smith-Bindman, R.; Yee, J.; Kuntz, K. M.; Ballegooijen, M.; Zauber, A. G.; Berg, C. D.
Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis Journal Article
In: AJR Am J Roentgenol, vol. 196, no. 4, pp. 816-23, 2011, ISSN: 1546-3141 (Electronic) 0361-803X, ().
@article{Gonzalez2011,
title = {Radiation-related cancer risks from CT colonography screening: a
risk-benefit analysis},
author = {A. Berrington Gonzalez and K. P. Kim and Amy Knudsen and I. Lansdorp-Vogelaar and C. M. Rutter and R. Smith-Bindman and J. Yee and K. M. Kuntz and M. Ballegooijen and A. G. Zauber and C. D. Berg},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21427330},
issn = {1546-3141 (Electronic) 0361-803X},
year = {2011},
date = {2011-04-01},
journal = {AJR Am J Roentgenol},
volume = {196},
number = {4},
pages = {816-23},
abstract = {OBJECTIVE: The purpose of this study was to estimate the ratio of
cancers prevented to induced (benefit-risk ratio) for CT colonography
(CTC) screening every 5 years from the age of 50 to 80 years. MATERIALS
AND METHODS: Radiation-related cancer risk was estimated using risk
projection models based on the National Research Council's Biological
Effects of Ionizing Radiation (BEIR) VII Committee's report and screening
protocols from the American College of Radiology Imaging Network's
National CT Colonography Trial. Uncertainty intervals were estimated
using Monte Carlo simulation methods. Comparative modeling with three
colorectal cancer microsimulation models was used to estimate the
potential reduction in colorectal cancer cases and deaths. RESULTS:
The estimated mean effective dose per CTC screening study was 8 mSv
for women and 7 mSv for men. The estimated number of radiation-related
cancers resulting from CTC screening every 5 years from the age of
50 to 80 years was 150 cases/100,000 individuals screened (95% uncertainty
interval, 80-280) for men and women. The estimated number of colorectal
cancers prevented by CTC every 5 years from age 50 to 80 ranged across
the three microsimulation models from 3580 to 5190 cases/100,000
individuals screened, yielding a benefit-risk ratio that varied from
24:1 (95% uncertainty interval, 13:1-45:1) to 35:1 (19:1-65:1). The
benefit-risk ratio for cancer deaths was even higher than the ratio
for cancer cases. Inclusion of radiation-related cancer risks from
CT examinations performed to follow up extracolonic findings did
not materially alter the results. CONCLUSION: Concerns have been
raised about recommending CTC as a routine screening tool because
of potential harms including the radiation risks. Based on these
models, the benefits from CTC screening every 5 years from the age
of 50 to 80 years clearly outweigh the radiation risks.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
cancers prevented to induced (benefit-risk ratio) for CT colonography
(CTC) screening every 5 years from the age of 50 to 80 years. MATERIALS
AND METHODS: Radiation-related cancer risk was estimated using risk
projection models based on the National Research Council's Biological
Effects of Ionizing Radiation (BEIR) VII Committee's report and screening
protocols from the American College of Radiology Imaging Network's
National CT Colonography Trial. Uncertainty intervals were estimated
using Monte Carlo simulation methods. Comparative modeling with three
colorectal cancer microsimulation models was used to estimate the
potential reduction in colorectal cancer cases and deaths. RESULTS:
The estimated mean effective dose per CTC screening study was 8 mSv
for women and 7 mSv for men. The estimated number of radiation-related
cancers resulting from CTC screening every 5 years from the age of
50 to 80 years was 150 cases/100,000 individuals screened (95% uncertainty
interval, 80-280) for men and women. The estimated number of colorectal
cancers prevented by CTC every 5 years from age 50 to 80 ranged across
the three microsimulation models from 3580 to 5190 cases/100,000
individuals screened, yielding a benefit-risk ratio that varied from
24:1 (95% uncertainty interval, 13:1-45:1) to 35:1 (19:1-65:1). The
benefit-risk ratio for cancer deaths was even higher than the ratio
for cancer cases. Inclusion of radiation-related cancer risks from
CT examinations performed to follow up extracolonic findings did
not materially alter the results. CONCLUSION: Concerns have been
raised about recommending CTC as a routine screening tool because
of potential harms including the radiation risks. Based on these
models, the benefits from CTC screening every 5 years from the age
of 50 to 80 years clearly outweigh the radiation risks.
Stock, C.; Knudsen, Amy; Lansdorp-Vogelaar, I.; Haug, U.; Brenner, H.
Colorectal cancer mortality prevented by use and attributable to nonuse of colonoscopy Journal Article
In: Gastrointest Endosc, vol. 73, pp. 435-443 e5, 2011, ().
@article{Stock2011,
title = {Colorectal cancer mortality prevented by use and attributable to
nonuse of colonoscopy},
author = {C. Stock and Amy Knudsen and I. Lansdorp-Vogelaar and U. Haug and H. Brenner},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21353840},
year = {2011},
date = {2011-01-01},
journal = {Gastrointest Endosc},
volume = {73},
pages = {435-443 e5},
abstract = {BACKGROUND: Use of colonoscopy is thought to reduce colorectal cancer
(CRC) mortality, but its impact at the population level is unclear.
OBJECTIVE: To estimate the effect of current colonoscopy use on CRC
mortality and its further potential in reducing CRC mortality. DESIGN:
Population-level analysis was performed by using the concepts of
prevented and attributable fractions, by using data from the National
Health Interview Survey, the Surveillance, Epidemiology and End Results
Program, and estimates of the effectiveness of colonoscopy at reducing
CRC mortality. SETTING: The 2005 U.S. population aged 50 years and
older. EXPOSURE: Colonoscopy within 10 years or less. MAIN OUTCOME
MEASUREMENTS: Percentages and absolute numbers of CRC deaths prevented
and potentially preventable by colonoscopy. LIMITATIONS: Uncertainty
in effectiveness estimates. RESULTS: Overall, the proportions of
CRC deaths in 2005 prevented by colonoscopy (ie, the prevented fractions)
range from 13% (95% CI, 1115 to 19% (95% CI, 1224 across the estimates
of colonoscopy effectiveness. Corresponding numbers of CRC deaths
prevented range from 7314 (95% CI, 6010-8467) to 11,711 (95% CI,
7077-14,898). The proportions of CRC deaths attributable to nonuse
of colonoscopy (ie, the attributable fractions) range from 28% (95%
CI, 2233 to 44% (95% CI, 2460, depending on the assumed effectiveness.
Corresponding numbers of CRC deaths attributed to nonuse of colonoscopy
range from 13,796 (95% CI, 11,076-16,255) to 22,088 (95% CI, 12,189-29,947).
CONCLUSIONS: Although we estimate that colonoscopy has prevented
substantial numbers of CRC deaths, many more deaths could have been
prevented with more widespread use. These findings highlight the
potential benefits from public health interventions to increase the
use of screening colonoscopy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
(CRC) mortality, but its impact at the population level is unclear.
OBJECTIVE: To estimate the effect of current colonoscopy use on CRC
mortality and its further potential in reducing CRC mortality. DESIGN:
Population-level analysis was performed by using the concepts of
prevented and attributable fractions, by using data from the National
Health Interview Survey, the Surveillance, Epidemiology and End Results
Program, and estimates of the effectiveness of colonoscopy at reducing
CRC mortality. SETTING: The 2005 U.S. population aged 50 years and
older. EXPOSURE: Colonoscopy within 10 years or less. MAIN OUTCOME
MEASUREMENTS: Percentages and absolute numbers of CRC deaths prevented
and potentially preventable by colonoscopy. LIMITATIONS: Uncertainty
in effectiveness estimates. RESULTS: Overall, the proportions of
CRC deaths in 2005 prevented by colonoscopy (ie, the prevented fractions)
range from 13% (95% CI, 1115 to 19% (95% CI, 1224 across the estimates
of colonoscopy effectiveness. Corresponding numbers of CRC deaths
prevented range from 7314 (95% CI, 6010-8467) to 11,711 (95% CI,
7077-14,898). The proportions of CRC deaths attributable to nonuse
of colonoscopy (ie, the attributable fractions) range from 28% (95%
CI, 2233 to 44% (95% CI, 2460, depending on the assumed effectiveness.
Corresponding numbers of CRC deaths attributed to nonuse of colonoscopy
range from 13,796 (95% CI, 11,076-16,255) to 22,088 (95% CI, 12,189-29,947).
CONCLUSIONS: Although we estimate that colonoscopy has prevented
substantial numbers of CRC deaths, many more deaths could have been
prevented with more widespread use. These findings highlight the
potential benefits from public health interventions to increase the
use of screening colonoscopy.
Ballegooijen, M.; Rutter, C. M.; Knudsen, Amy; Zauber, A. G.; Savarino, J. E.; Lansdorp-Vogelaar, I.; Boer, R.; Feuer, E. J.; Habbema, J. D.; Kuntz, K. M.
Clarifying differences in natural history between models of screening: the case of colorectal cancer Journal Article
In: Med Decis Making, vol. 31, pp. 540-9, 2011, ().
@article{Ballegooijen2011,
title = {Clarifying differences in natural history between models of screening:
the case of colorectal cancer},
author = {M. Ballegooijen and C. M. Rutter and Amy Knudsen and A. G. Zauber and J. E. Savarino and I. Lansdorp-Vogelaar and R. Boer and E. J. Feuer and J. D. Habbema and K. M. Kuntz},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21673187},
year = {2011},
date = {2011-01-01},
journal = {Med Decis Making},
volume = {31},
pages = {540-9},
abstract = {BACKGROUND: Microsimulation models are important decision support
tools for screening. However, their complexity makes them difficult
to understand and limits realization of their full potential. Therefore,
it is important to develop documentation that clarifies their structure
and assumptions. The authors demonstrate this problem and explore
a solution for natural history using 3 independently developed colorectal
cancer screening models. METHODS: The authors first project effectiveness
and cost-effectiveness of colonoscopy screening for the 3 models
(CRC-SPIN, SimCRC, and MISCAN). Next, they provide a conventional
presentation of each model, including information on structure and
parameter values. Finally, they report the simulated reduction in
clinical cancer incidence following a one-time complete removal of
adenomas and preclinical cancers for each model. They call this new
measure the maximum clinical incidence reduction (MCLIR). RESULTS:
Projected effectiveness varies widely across models. For example,
estimated mortality reduction for colonoscopy screening every 10
years from age 50 to 80 years, with surveillance in adenoma patients,
ranges from 65% to 92 Given only conventional information, it is
difficult to explain these differences, largely because differences
in structure make parameter values incomparable. In contrast, the
MCLIR clearly shows the impact of model differences on the key feature
of natural history, the dwell time of preclinical disease. Dwell
times vary from 8 to 25 years across models and help explain differences
in projected screening effectiveness. CONCLUSIONS: The authors propose
a new measure, the MCLIR, which summarizes the implications for predicted
screening effectiveness of differences in natural history assumptions.
Including the MCLIR in the standard description of a screening model
would improve the transparency of these models.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
tools for screening. However, their complexity makes them difficult
to understand and limits realization of their full potential. Therefore,
it is important to develop documentation that clarifies their structure
and assumptions. The authors demonstrate this problem and explore
a solution for natural history using 3 independently developed colorectal
cancer screening models. METHODS: The authors first project effectiveness
and cost-effectiveness of colonoscopy screening for the 3 models
(CRC-SPIN, SimCRC, and MISCAN). Next, they provide a conventional
presentation of each model, including information on structure and
parameter values. Finally, they report the simulated reduction in
clinical cancer incidence following a one-time complete removal of
adenomas and preclinical cancers for each model. They call this new
measure the maximum clinical incidence reduction (MCLIR). RESULTS:
Projected effectiveness varies widely across models. For example,
estimated mortality reduction for colonoscopy screening every 10
years from age 50 to 80 years, with surveillance in adenoma patients,
ranges from 65% to 92 Given only conventional information, it is
difficult to explain these differences, largely because differences
in structure make parameter values incomparable. In contrast, the
MCLIR clearly shows the impact of model differences on the key feature
of natural history, the dwell time of preclinical disease. Dwell
times vary from 8 to 25 years across models and help explain differences
in projected screening effectiveness. CONCLUSIONS: The authors propose
a new measure, the MCLIR, which summarizes the implications for predicted
screening effectiveness of differences in natural history assumptions.
Including the MCLIR in the standard description of a screening model
would improve the transparency of these models.
Kuntz, K. M.; Lansdorp-Vogelaar, I.; Rutter, C. M.; Knudsen, Amy; Ballegooijen, M.; Savarino, J. E.; Feuer, E. J.; Zauber, A. G.
A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression Journal Article
In: Med Decis Making, vol. 31, pp. 530-9, 2011, ().
@article{Kuntz2011,
title = {A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression},
author = {K. M. Kuntz and I. Lansdorp-Vogelaar and C. M. Rutter and Amy Knudsen and M. Ballegooijen and J. E. Savarino and E. J. Feuer and A. G. Zauber},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21673186},
year = {2011},
date = {2011-01-01},
urldate = {2011-01-01},
journal = {Med Decis Making},
volume = {31},
pages = {530-9},
abstract = {BACKGROUND: As the complexity of microsimulation models increases,
concerns about model transparency are heightened. METHODS: The authors
conducted model "experiments" to explore the impact of variations
in "deep" model parameters using 3 colorectal cancer (CRC) models.
All natural history models were calibrated to match observed data
on adenoma prevalence and cancer incidence but varied in their underlying
specification of the adenocarcinoma process. The authors projected
CRC incidence among individuals with an underlying adenoma or preclinical
cancer v. those without any underlying condition and examined the
impact of removing adenomas. They calculated the percentage of simulated
CRC cases arising from adenomas that developed within 10 or 20 years
prior to cancer diagnosis and estimated dwell time-defined as the
time from the development of an adenoma to symptom-detected cancer
in the absence of screening among individuals with a CRC diagnosis.
RESULTS: The 20-year CRC incidence among 55-year-old individuals
with an adenoma or preclinical cancer was 7 to 75 times greater than
in the condition-free group. The removal of all adenomas among the
subgroup with an underlying adenoma or cancer resulted in a reduction
of 30% to 89% in cumulative incidence. Among CRCs diagnosed at age
65 years, the proportion arising from adenomas formed within 10 years
ranged between 4% and 67 The mean dwell time varied from 10.6 to
25.8 years. CONCLUSIONS: Models that all match observed data on adenoma
prevalence and cancer incidence can produce quite different dwell
times and very different answers with respect to the effectiveness
of interventions. When conducting applied analyses to inform policy,
using multiple models provides a sensitivity analysis on key (unobserved)
"deep" model parameters and can provide guidance about specific areas
in need of additional research and validation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
concerns about model transparency are heightened. METHODS: The authors
conducted model "experiments" to explore the impact of variations
in "deep" model parameters using 3 colorectal cancer (CRC) models.
All natural history models were calibrated to match observed data
on adenoma prevalence and cancer incidence but varied in their underlying
specification of the adenocarcinoma process. The authors projected
CRC incidence among individuals with an underlying adenoma or preclinical
cancer v. those without any underlying condition and examined the
impact of removing adenomas. They calculated the percentage of simulated
CRC cases arising from adenomas that developed within 10 or 20 years
prior to cancer diagnosis and estimated dwell time-defined as the
time from the development of an adenoma to symptom-detected cancer
in the absence of screening among individuals with a CRC diagnosis.
RESULTS: The 20-year CRC incidence among 55-year-old individuals
with an adenoma or preclinical cancer was 7 to 75 times greater than
in the condition-free group. The removal of all adenomas among the
subgroup with an underlying adenoma or cancer resulted in a reduction
of 30% to 89% in cumulative incidence. Among CRCs diagnosed at age
65 years, the proportion arising from adenomas formed within 10 years
ranged between 4% and 67 The mean dwell time varied from 10.6 to
25.8 years. CONCLUSIONS: Models that all match observed data on adenoma
prevalence and cancer incidence can produce quite different dwell
times and very different answers with respect to the effectiveness
of interventions. When conducting applied analyses to inform policy,
using multiple models provides a sensitivity analysis on key (unobserved)
"deep" model parameters and can provide guidance about specific areas
in need of additional research and validation.
Haug, U.; Knudsen, Amy; Brenner, H.; Kuntz, K. M.
Is fecal occult blood testing more sensitive for left- versus right-sided colorectal neoplasia? A systematic literature review Journal Article
In: Expert Rev Mol Diagn, vol. 11, pp. 605–16, 2011, ISSN: 1744-8352 (Electronic) 1473-7159, ().
@article{haug_is_2011,
title = {Is fecal occult blood testing more sensitive for left- versus right-sided colorectal neoplasia? A systematic literature review},
author = {U. Haug and Amy Knudsen and H. Brenner and K. M. Kuntz},
url = {http://www.ncbi.nlm.nih.gov/pubmed/21745014},
doi = {Research Support, Non-U.S. Gov't Review},
issn = {1744-8352 (Electronic) 1473-7159},
year = {2011},
date = {2011-01-01},
urldate = {2011-01-01},
journal = {Expert Rev Mol Diagn},
volume = {11},
pages = {605--16},
abstract = {Owing to its slow development from removable precursor lesions and early cancer stages with good prognosis, screening for colorectal cancer holds potential to reduce both the incidence and mortality of the disease. While sigmoidoscopy only detects left-sided neoplasia, there is accumulating evidence that colonoscopy is also more effective in protecting from neoplasia in the left versus the right colon and rectum. In this context, it is an important question whether the sensitivity of the most common noninvasive screening tool for colorectal cancer, fecal occult blood testing (FOBT), also differs for left- versus right-sided neoplasia. Therefore, we systematically searched the literature for prospective screening studies conducted in average-risk adults that performed FOBT (immunochemical and/or guaiac-based) and colonoscopy among all participants, and reported site-specific sensitivities of FOBT for advanced colorectal neoplasia. Most of the seven included studies showed a higher sensitivity of FOBT for advanced neoplasia in the left versus right colon, but this finding needs to be confirmed since the available literature is scarce and not entirely consistent.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2010
Lansdorp-Vogelaar, I.; Kuntz, K. M.; Knudsen, Amy; Wilschut, J. A.; Zauber, A. G.; Ballegooijen, M.
Stool DNA testing to screen for colorectal cancer in the Medicare population: a cost-effectiveness analysis Journal Article
In: Ann Intern Med, vol. 153, no. 6, pp. 368-77, 2010, ISSN: 1539-3704 (Electronic) 0003-4819, ().
@article{Lansdorp-Vogelaar2010,
title = {Stool DNA testing to screen for colorectal cancer in the Medicare
population: a cost-effectiveness analysis},
author = {I. Lansdorp-Vogelaar and K. M. Kuntz and Amy Knudsen and J. A. Wilschut and A. G. Zauber and M. Ballegooijen},
url = {http://www.ncbi.nlm.nih.gov/pubmed/20855801},
issn = {1539-3704 (Electronic) 0003-4819},
year = {2010},
date = {2010-09-01},
journal = {Ann Intern Med},
volume = {153},
number = {6},
pages = {368-77},
abstract = {BACKGROUND: The Centers for Medicare \& Medicaid Services considered
whether to reimburse stool DNA testing for colorectal cancer screening
among Medicare enrollees. OBJECTIVE: To evaluate the conditions under
which stool DNA testing could be cost-effective compared with the
colorectal cancer screening tests currently reimbursed by the Centers
for Medicare \& Medicaid Services. DESIGN: Comparative microsimulation
modeling study using 2 independently developed models. DATA SOURCES:
Derived from literature. TARGET POPULATION: A cohort of persons aged
65 years. A sensitivity analysis was also conducted, in which a cohort
of persons aged 50 years was studied. TIME HORIZON: Lifetime. Perspective:
Third-party payer. Intervention: Stool DNA test every 3 or 5 years
in comparison with currently recommended colorectal cancer screening
strategies. OUTCOME MEASURES: Life expectancy, lifetime costs, incremental
cost-effectiveness ratios, and threshold costs. RESULTS OF BASE-CASE
ANALYSIS: Assuming a cost of $350 per test, strategies of stool DNA
testing every 3 or 5 years yielded fewer life-years and higher costs
than the currently recommended colorectal cancer screening strategies.
Screening with the stool DNA test would be cost-effective at a per-test
cost of $40 to $60 for stool DNA testing every 3 years, depending
on the simulation model used. There were no levels of sensitivity
and specificity for which stool DNA testing would be cost-effective
at its current cost of $350 per test. Stool DNA testing every 3 years
would be cost-effective at a cost of $350 per test if the relative
adherence to stool DNA testing were at least 50% better than that
with other screening tests. RESULTS OF SENSITIVITY ANALYSIS: None
of the results changed substantially when a cohort of persons aged
50 years was considered. LIMITATION: No pathways other than the traditional
adenoma-carcinoma sequence were modeled. CONCLUSION: Stool DNA testing
could be a cost-effective alternative for colorectal cancer screening
if the cost of the test substantially decreased or if its availability
would entice a large fraction of otherwise unscreened persons to
receive screening.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
whether to reimburse stool DNA testing for colorectal cancer screening
among Medicare enrollees. OBJECTIVE: To evaluate the conditions under
which stool DNA testing could be cost-effective compared with the
colorectal cancer screening tests currently reimbursed by the Centers
for Medicare & Medicaid Services. DESIGN: Comparative microsimulation
modeling study using 2 independently developed models. DATA SOURCES:
Derived from literature. TARGET POPULATION: A cohort of persons aged
65 years. A sensitivity analysis was also conducted, in which a cohort
of persons aged 50 years was studied. TIME HORIZON: Lifetime. Perspective:
Third-party payer. Intervention: Stool DNA test every 3 or 5 years
in comparison with currently recommended colorectal cancer screening
strategies. OUTCOME MEASURES: Life expectancy, lifetime costs, incremental
cost-effectiveness ratios, and threshold costs. RESULTS OF BASE-CASE
ANALYSIS: Assuming a cost of $350 per test, strategies of stool DNA
testing every 3 or 5 years yielded fewer life-years and higher costs
than the currently recommended colorectal cancer screening strategies.
Screening with the stool DNA test would be cost-effective at a per-test
cost of $40 to $60 for stool DNA testing every 3 years, depending
on the simulation model used. There were no levels of sensitivity
and specificity for which stool DNA testing would be cost-effective
at its current cost of $350 per test. Stool DNA testing every 3 years
would be cost-effective at a cost of $350 per test if the relative
adherence to stool DNA testing were at least 50% better than that
with other screening tests. RESULTS OF SENSITIVITY ANALYSIS: None
of the results changed substantially when a cohort of persons aged
50 years was considered. LIMITATION: No pathways other than the traditional
adenoma-carcinoma sequence were modeled. CONCLUSION: Stool DNA testing
could be a cost-effective alternative for colorectal cancer screening
if the cost of the test substantially decreased or if its availability
would entice a large fraction of otherwise unscreened persons to
receive screening.
Knudsen, Amy; Lansdorp-Vogelaar, I.; Rutter, C. M.; Savarino, J. E.; Ballegooijen, M.; Kuntz, K. M.; Zauber, A. G.
Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population Journal Article
In: J Natl Cancer Inst, vol. 102, no. 16, pp. 1238-52, 2010, ISSN: 1460-2105 (Electronic) 0027-8874, ().
@article{Knudsen2010,
title = {Cost-effectiveness of computed tomographic colonography screening
for colorectal cancer in the medicare population},
author = {Amy Knudsen and I. Lansdorp-Vogelaar and C. M. Rutter and J. E. Savarino and M. Ballegooijen and K. M. Kuntz and A. G. Zauber},
url = {http://www.ncbi.nlm.nih.gov/pubmed/20664028},
issn = {1460-2105 (Electronic) 0027-8874},
year = {2010},
date = {2010-08-01},
journal = {J Natl Cancer Inst},
volume = {102},
number = {16},
pages = {1238-52},
abstract = {BACKGROUND: The Centers for Medicare and Medicaid Services (CMS) considered
whether to reimburse computed tomographic colonography (CTC) for
colorectal cancer screening of Medicare enrollees. To help inform
its decision, we evaluated the reimbursement rate at which CTC screening
could be cost-effective compared with the colorectal cancer screening
tests that are currently reimbursed by CMS and are included in most
colorectal cancer screening guidelines, namely annual fecal occult
blood test (FOBT), flexible sigmoidoscopy every 5 years, flexible
sigmoidoscopy every 5 years in conjunction with annual FOBT, and
colonoscopy every 10 years. METHODS: We used three independently
developed microsimulation models to assess the health outcomes and
costs associated with CTC screening and with currently reimbursed
colorectal cancer screening tests among the average-risk Medicare
population. We assumed that CTC was performed every 5 years (using
test characteristics from either a Department of Defense CTC study
or the National CTC Trial) and that individuals with findings of
6 mm or larger were referred to colonoscopy. We computed incremental
cost-effectiveness ratios for the currently reimbursed screening
tests and calculated the maximum cost per scan (ie, the threshold
cost) for the CTC strategy to lie on the efficient frontier. Sensitivity
analyses were performed on key parameters and assumptions. RESULTS:
Assuming perfect adherence with all tests, the undiscounted number
life-years gained from CTC screening ranged from 143 to 178 per 1000
65-year-olds, which was slightly less than the number of life-years
gained from 10-yearly colonoscopy (152-185 per 1000 65-year-olds)
and comparable to that from 5-yearly sigmoidoscopy with annual FOBT
(149-177 per 1000 65-year-olds). If CTC screening was reimbursed
at $488 per scan (slightly less than the reimbursement for a colonoscopy
without polypectomy), it would be the most costly strategy. CTC screening
could be cost-effective at $108-$205 per scan, depending on the microsimulation
model used. Sensitivity analyses showed that if relative adherence
to CTC screening was 25% higher than adherence to other tests, it
could be cost-effective if reimbursed at $488 per scan. CONCLUSIONS:
CTC could be a cost-effective option for colorectal cancer screening
among Medicare enrollees if the reimbursement rate per scan is substantially
less than that for colonoscopy or if a large proportion of otherwise
unscreened persons were to undergo screening by CTC.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
whether to reimburse computed tomographic colonography (CTC) for
colorectal cancer screening of Medicare enrollees. To help inform
its decision, we evaluated the reimbursement rate at which CTC screening
could be cost-effective compared with the colorectal cancer screening
tests that are currently reimbursed by CMS and are included in most
colorectal cancer screening guidelines, namely annual fecal occult
blood test (FOBT), flexible sigmoidoscopy every 5 years, flexible
sigmoidoscopy every 5 years in conjunction with annual FOBT, and
colonoscopy every 10 years. METHODS: We used three independently
developed microsimulation models to assess the health outcomes and
costs associated with CTC screening and with currently reimbursed
colorectal cancer screening tests among the average-risk Medicare
population. We assumed that CTC was performed every 5 years (using
test characteristics from either a Department of Defense CTC study
or the National CTC Trial) and that individuals with findings of
6 mm or larger were referred to colonoscopy. We computed incremental
cost-effectiveness ratios for the currently reimbursed screening
tests and calculated the maximum cost per scan (ie, the threshold
cost) for the CTC strategy to lie on the efficient frontier. Sensitivity
analyses were performed on key parameters and assumptions. RESULTS:
Assuming perfect adherence with all tests, the undiscounted number
life-years gained from CTC screening ranged from 143 to 178 per 1000
65-year-olds, which was slightly less than the number of life-years
gained from 10-yearly colonoscopy (152-185 per 1000 65-year-olds)
and comparable to that from 5-yearly sigmoidoscopy with annual FOBT
(149-177 per 1000 65-year-olds). If CTC screening was reimbursed
at $488 per scan (slightly less than the reimbursement for a colonoscopy
without polypectomy), it would be the most costly strategy. CTC screening
could be cost-effective at $108-$205 per scan, depending on the microsimulation
model used. Sensitivity analyses showed that if relative adherence
to CTC screening was 25% higher than adherence to other tests, it
could be cost-effective if reimbursed at $488 per scan. CONCLUSIONS:
CTC could be a cost-effective option for colorectal cancer screening
among Medicare enrollees if the reimbursement rate per scan is substantially
less than that for colonoscopy or if a large proportion of otherwise
unscreened persons were to undergo screening by CTC.
Lansdorp-Vogelaar, I.; Knudsen, Amy; Brenner, H.
Cost-effectiveness of colorectal cancer screening - an overview Journal Article
In: Best Pract Res Clin Gastroenterol, vol. 24, no. 4, pp. 439-49, 2010, ISSN: 1532-1916 (Electronic) 1521-6918, ().
@article{Lansdorp-Vogelaar2010a,
title = {Cost-effectiveness of colorectal cancer screening - an overview},
author = {I. Lansdorp-Vogelaar and Amy Knudsen and H. Brenner},
url = {http://www.ncbi.nlm.nih.gov/pubmed/20833348},
issn = {1532-1916 (Electronic) 1521-6918},
year = {2010},
date = {2010-08-01},
journal = {Best Pract Res Clin Gastroenterol},
volume = {24},
number = {4},
pages = {439-49},
abstract = {There are several modalities available for a colorectal cancer (CRC)
screening program. When determining which CRC screening program to
implement, the costs of such programs should be considered in comparison
to the health benefits they are expected to provide. Cost-effectiveness
analysis provides a tool to do this. In this paper we review the
evidence on the cost-effectiveness of CRC screening. Published studies
universally indicate that when compared with no CRC screening, all
screening modalities provide additional years of life at a cost that
is deemed acceptable by most industrialized nations. Many recent
studies even find CRC screening to be cost-saving. However, when
the alternative CRC screening strategies are compared against each
other in an incremental cost-effectiveness analysis, no single optimal
strategy emerges across the studies. There is consensus that the
new technologies of stool DNA testing, computed tomographic colonography
and capsule endoscopy are not yet cost-effective compared with the
established CRC screening tests.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
screening program. When determining which CRC screening program to
implement, the costs of such programs should be considered in comparison
to the health benefits they are expected to provide. Cost-effectiveness
analysis provides a tool to do this. In this paper we review the
evidence on the cost-effectiveness of CRC screening. Published studies
universally indicate that when compared with no CRC screening, all
screening modalities provide additional years of life at a cost that
is deemed acceptable by most industrialized nations. Many recent
studies even find CRC screening to be cost-saving. However, when
the alternative CRC screening strategies are compared against each
other in an incremental cost-effectiveness analysis, no single optimal
strategy emerges across the studies. There is consensus that the
new technologies of stool DNA testing, computed tomographic colonography
and capsule endoscopy are not yet cost-effective compared with the
established CRC screening tests.
2009
Greenblatt, Wesley; Hur, Chin; Knudsen, Amy; Evans, J. A.; Chung, D. C.; Gazelle, G. Scott
Cost-effectiveness of prophylactic surgery for duodenal cancer in familial adenomatous polyposis Journal Article
In: Cancer Epidemiol Biomarkers Prev, vol. 18, no. 10, pp. 2677-84, 2009, ISSN: 1538-7755 (Electronic) 1055-9965, ().
@article{Greenblatt2009,
title = {Cost-effectiveness of prophylactic surgery for duodenal cancer in
familial adenomatous polyposis},
author = {Wesley Greenblatt and Chin Hur and Amy Knudsen and J. A. Evans and D. C. Chung and G. Scott Gazelle},
url = {http://www.ncbi.nlm.nih.gov/pubmed/19789369},
issn = {1538-7755 (Electronic) 1055-9965},
year = {2009},
date = {2009-10-01},
journal = {Cancer Epidemiol Biomarkers Prev},
volume = {18},
number = {10},
pages = {2677-84},
abstract = {BACKGROUND: Duodenal cancer is the leading cause of cancer death in
familial adenomatous polyposis after colorectal cancer. The lifetime
risk for developing duodenal cancer is 4% to 10%. Current treatment
guidelines recommend endoscopic surveillance with a prophylactic
pancreaticoduodenectomy in advanced duodenal polyposis, defined using
the Spigelman staging system. Because no clinical trials have assessed
this recommendation, a modeling approach was used to evaluate the
cost-effectiveness of various treatment strategies. METHODS: A Markov
model was constructed to estimate the life expectancy and cost of
three different strategies: pancreaticoduodenectomy at Spigelman
stage III, pancreaticoduodenectomy at Spigelman stage IV, and pancreaticoduodenectomy
at cancer diagnosis. A cohort of 30-year-old familial adenomatous
polyposis patients with total colectomies was simulated until age
80. The analysis was from a societal perspective. Extensive sensitivity
analysis was performed to assess the impact of model uncertainty
on results. RESULTS: At all stages of polyposis and all ages \<80
years, prophylactic surgery at Spigelman stage IV resulted in the
greatest life expectancy. Surgery at stage IV was more effective
and more expensive than surgery at cancer diagnosis, with an incremental
cost of $3,200 per quality-adjusted life year gained. Surgery at
stage III was not a viable option. The results were robust to wide
variation in model parameters but were sensitive to the post-pancreaticoduodenectomy
quality of life score. CONCLUSIONS: Prophylactic pancreaticoduodenectomy
at stage IV duodenal polyposis in familial adenomatous polyposis
is a cost-effective approach that results in greater life expectancy
than surgery at either stage III or cancer diagnosis.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
familial adenomatous polyposis after colorectal cancer. The lifetime
risk for developing duodenal cancer is 4% to 10%. Current treatment
guidelines recommend endoscopic surveillance with a prophylactic
pancreaticoduodenectomy in advanced duodenal polyposis, defined using
the Spigelman staging system. Because no clinical trials have assessed
this recommendation, a modeling approach was used to evaluate the
cost-effectiveness of various treatment strategies. METHODS: A Markov
model was constructed to estimate the life expectancy and cost of
three different strategies: pancreaticoduodenectomy at Spigelman
stage III, pancreaticoduodenectomy at Spigelman stage IV, and pancreaticoduodenectomy
at cancer diagnosis. A cohort of 30-year-old familial adenomatous
polyposis patients with total colectomies was simulated until age
80. The analysis was from a societal perspective. Extensive sensitivity
analysis was performed to assess the impact of model uncertainty
on results. RESULTS: At all stages of polyposis and all ages <80
years, prophylactic surgery at Spigelman stage IV resulted in the
greatest life expectancy. Surgery at stage IV was more effective
and more expensive than surgery at cancer diagnosis, with an incremental
cost of $3,200 per quality-adjusted life year gained. Surgery at
stage III was not a viable option. The results were robust to wide
variation in model parameters but were sensitive to the post-pancreaticoduodenectomy
quality of life score. CONCLUSIONS: Prophylactic pancreaticoduodenectomy
at stage IV duodenal polyposis in familial adenomatous polyposis
is a cost-effective approach that results in greater life expectancy
than surgery at either stage III or cancer diagnosis.
Stout, N. K.; Knudsen, Amy; Kong, Chung Yin; McMahon, Pamela M.; Gazelle, G. Scott
Calibration methods used in cancer simulation models and suggested reporting guidelines Journal Article
In: PharmacoEconomics, vol. 27, pp. 533-45, 2009, ().
@article{Stout2009,
title = {Calibration methods used in cancer simulation models and suggested
reporting guidelines},
author = {N. K. Stout and Amy Knudsen and Chung Yin Kong and Pamela M. McMahon and G. Scott Gazelle},
url = {http://www.ncbi.nlm.nih.gov/pubmed/19663525},
year = {2009},
date = {2009-01-01},
journal = {PharmacoEconomics},
volume = {27},
pages = {533-45},
abstract = {Increasingly, computer simulation models are used for economic and
policy evaluation in cancer prevention and control. A model's predictions
of key outcomes, such as screening effectiveness, depend on the values
of unobservable natural history parameters. Calibration is the process
of determining the values of unobservable parameters by constraining
model output to replicate observed data. Because there are many approaches
for model calibration and little consensus on best practices, we
surveyed the literature to catalogue the use and reporting of these
methods in cancer simulation models. We conducted a MEDLINE search
(1980 through 2006) for articles on cancer-screening models and supplemented
search results with articles from our personal reference databases.
For each article, two authors independently abstracted pre-determined
items using a standard form. Data items included cancer site, model
type, methods used for determination of unobservable parameter values
and description of any calibration protocol. All authors reached
consensus on items of disagreement. Reviews and non-cancer models
were excluded. Articles describing analytical models, which estimate
parameters with statistical approaches (e.g. maximum likelihood)
were catalogued separately. Models that included unobservable parameters
were analysed and classified by whether calibration methods were
reported and if so, the methods used. The review process yielded
154 articles that met our inclusion criteria and, of these, we concluded
that 131 may have used calibration methods to determine model parameters.
Although the term 'calibration' was not always used, descriptions of calibration or 'model fitting' were found in 50% (n = 66) of the articles, with an additional 16% (n = 21) providing a reference to
methods. Calibration target data were identified in nearly all of
these articles. Other methodological details, such as the goodness-of-fit metric, were discussed in 54% (n = 47 of 87) of the articles reporting
calibration methods, while few details were provided on the algorithms
used to search the parameter space. Our review shows that the use
of cancer simulation modelling is increasing, although thorough descriptions
of calibration procedures are rare in the published literature for
these models. Calibration is a key component of model development
and is central to the validity and credibility of subsequent analyses
and inferences drawn from model predictions. To aid peer-review and
facilitate discussion of modelling methods, we propose a standardized
Calibration Reporting Checklist for model documentation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
policy evaluation in cancer prevention and control. A model's predictions
of key outcomes, such as screening effectiveness, depend on the values
of unobservable natural history parameters. Calibration is the process
of determining the values of unobservable parameters by constraining
model output to replicate observed data. Because there are many approaches
for model calibration and little consensus on best practices, we
surveyed the literature to catalogue the use and reporting of these
methods in cancer simulation models. We conducted a MEDLINE search
(1980 through 2006) for articles on cancer-screening models and supplemented
search results with articles from our personal reference databases.
For each article, two authors independently abstracted pre-determined
items using a standard form. Data items included cancer site, model
type, methods used for determination of unobservable parameter values
and description of any calibration protocol. All authors reached
consensus on items of disagreement. Reviews and non-cancer models
were excluded. Articles describing analytical models, which estimate
parameters with statistical approaches (e.g. maximum likelihood)
were catalogued separately. Models that included unobservable parameters
were analysed and classified by whether calibration methods were
reported and if so, the methods used. The review process yielded
154 articles that met our inclusion criteria and, of these, we concluded
that 131 may have used calibration methods to determine model parameters.
Although the term 'calibration' was not always used, descriptions of calibration or 'model fitting' were found in 50% (n = 66) of the articles, with an additional 16% (n = 21) providing a reference to
methods. Calibration target data were identified in nearly all of
these articles. Other methodological details, such as the goodness-of-fit metric, were discussed in 54% (n = 47 of 87) of the articles reporting
calibration methods, while few details were provided on the algorithms
used to search the parameter space. Our review shows that the use
of cancer simulation modelling is increasing, although thorough descriptions
of calibration procedures are rare in the published literature for
these models. Calibration is a key component of model development
and is central to the validity and credibility of subsequent analyses
and inferences drawn from model predictions. To aid peer-review and
facilitate discussion of modelling methods, we propose a standardized
Calibration Reporting Checklist for model documentation.
2008
Zauber, A. G.; Lansdorp-Vogelaar, I.; Knudsen, Amy; Wilschut, J.; Ballegooijen, M.; Kuntz, K. M.
Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force Journal Article
In: Ann Intern Med, vol. 149, no. 9, pp. 659-69, 2008, ISSN: 1539-3704 (Electronic) 0003-4819, ().
@article{Zauber2008,
title = {Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force},
author = {A. G. Zauber and I. Lansdorp-Vogelaar and Amy Knudsen and J. Wilschut and M. Ballegooijen and K. M. Kuntz},
url = {http://www.ncbi.nlm.nih.gov/pubmed/18838717},
issn = {1539-3704 (Electronic) 0003-4819},
year = {2008},
date = {2008-11-01},
urldate = {2008-11-01},
journal = {Ann Intern Med},
volume = {149},
number = {9},
pages = {659-69},
abstract = {BACKGROUND: The U.S. Preventive Services Task Force requested a decision
analysis to inform their update of recommendations for colorectal
cancer screening. OBJECTIVE: To assess life-years gained and colonoscopy
requirements for colorectal cancer screening strategies and identify
a set of recommendable screening strategies. DESIGN: Decision analysis
using 2 colorectal cancer microsimulation models from the Cancer
Intervention and Surveillance Modeling Network. DATA SOURCES: Derived
from the literature. TARGET POPULATION: U.S. average-risk 40-year-old
population. PERSPECTIVE: Societal. TIME HORIZON: Lifetime. INTERVENTIONS:
Fecal occult blood tests (FOBTs), flexible sigmoidoscopy, or colonoscopy
screening beginning at age 40, 50, or 60 years and stopping at age
75 or 85 years, with screening intervals of 1, 2, or 3 years for
FOBT and 5, 10, or 20 years for sigmoidoscopy and colonoscopy. Outcome
Measures: Number of life-years gained compared with no screening
and number of colonoscopies and noncolonoscopy tests required. RESULTS
OF BASE-CASE ANALYSIS: Beginning screening at age 50 years was consistently
better than at age 60. Decreasing the stop age from 85 to 75 years
decreased life-years gained by 1% to 4%, whereas colonoscopy use
decreased by 4% to 15%. Assuming equally high adherence, 4 strategies
provided similar life-years gained: colonoscopy every 10 years, annual
Hemoccult SENSA (Beckman Coulter, Fullerton, California) testing
or fecal immunochemical testing, and sigmoidoscopy every 5 years
with midinterval Hemoccult SENSA testing. Annual Hemoccult II and
flexible sigmoidoscopy every 5 years alone were less effective. RESULTS
OF SENSITIVITY ANALYSIS: The results were most sensitive to beginning
screening at age 40 years. Limitation: The stop age for screening
was based only on chronologic age. CONCLUSION: The findings support
colorectal cancer screening with the following: colonoscopy every
10 years, annual screening with a sensitive FOBT, or flexible sigmoidoscopy
every 5 years with a midinterval sensitive FOBT from age 50 to 75
years.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
analysis to inform their update of recommendations for colorectal
cancer screening. OBJECTIVE: To assess life-years gained and colonoscopy
requirements for colorectal cancer screening strategies and identify
a set of recommendable screening strategies. DESIGN: Decision analysis
using 2 colorectal cancer microsimulation models from the Cancer
Intervention and Surveillance Modeling Network. DATA SOURCES: Derived
from the literature. TARGET POPULATION: U.S. average-risk 40-year-old
population. PERSPECTIVE: Societal. TIME HORIZON: Lifetime. INTERVENTIONS:
Fecal occult blood tests (FOBTs), flexible sigmoidoscopy, or colonoscopy
screening beginning at age 40, 50, or 60 years and stopping at age
75 or 85 years, with screening intervals of 1, 2, or 3 years for
FOBT and 5, 10, or 20 years for sigmoidoscopy and colonoscopy. Outcome
Measures: Number of life-years gained compared with no screening
and number of colonoscopies and noncolonoscopy tests required. RESULTS
OF BASE-CASE ANALYSIS: Beginning screening at age 50 years was consistently
better than at age 60. Decreasing the stop age from 85 to 75 years
decreased life-years gained by 1% to 4%, whereas colonoscopy use
decreased by 4% to 15%. Assuming equally high adherence, 4 strategies
provided similar life-years gained: colonoscopy every 10 years, annual
Hemoccult SENSA (Beckman Coulter, Fullerton, California) testing
or fecal immunochemical testing, and sigmoidoscopy every 5 years
with midinterval Hemoccult SENSA testing. Annual Hemoccult II and
flexible sigmoidoscopy every 5 years alone were less effective. RESULTS
OF SENSITIVITY ANALYSIS: The results were most sensitive to beginning
screening at age 40 years. Limitation: The stop age for screening
was based only on chronologic age. CONCLUSION: The findings support
colorectal cancer screening with the following: colonoscopy every
10 years, annual screening with a sensitive FOBT, or flexible sigmoidoscopy
every 5 years with a midinterval sensitive FOBT from age 50 to 75
years.
Pearson, Steven; Knudsen, Amy; Scherer, R. W.; Weissberg, J.; Gazelle, G. Scott
Assessing the comparative effectiveness of a diagnostic technology: CT colonography Journal Article
In: Health Aff (Millwood), vol. 27, no. 6, pp. 1503-14, 2008, ISSN: 1544-5208 (Electronic) 0278-2715, ().
@article{Pearson2008a,
title = {Assessing the comparative effectiveness of a diagnostic technology: CT colonography},
author = {Steven Pearson and Amy Knudsen and R. W. Scherer and J. Weissberg and G. Scott Gazelle},
url = {http://www.ncbi.nlm.nih.gov/pubmed/18997205},
issn = {1544-5208 (Electronic) 0278-2715},
year = {2008},
date = {2008-00-01},
urldate = {2008-00-01},
journal = {Health Aff (Millwood)},
volume = {27},
number = {6},
pages = {1503-14},
abstract = {Medical imaging is a prime example of an innovation that has brought
important advances to medical care while triggering concerns about
potential overuse and excessive costs. Many hopes are riding on comparative
effectiveness research to help guide better decision making to improve
quality and value. But the dynamic nature of medical imaging poses
challenges for the traditional paradigms of evidentiary review and
analysis at the heart of comparative effectiveness. This paper discusses
these challenges and presents policy lessons for manufacturers, evidence
reviewers, and decisionmakers, illustrated by an assessment of a
prominent emerging imaging technique: computed tomography (CT) colonography.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
important advances to medical care while triggering concerns about
potential overuse and excessive costs. Many hopes are riding on comparative
effectiveness research to help guide better decision making to improve
quality and value. But the dynamic nature of medical imaging poses
challenges for the traditional paradigms of evidentiary review and
analysis at the heart of comparative effectiveness. This paper discusses
these challenges and presents policy lessons for manufacturers, evidence
reviewers, and decisionmakers, illustrated by an assessment of a
prominent emerging imaging technique: computed tomography (CT) colonography.
2007
Knudsen, Amy; McMahon, Pamela M.; Gazelle, G. Scott
Use of modeling to evaluate the cost-effectiveness of cancer screening programs Journal Article
In: J Clin Oncol, vol. 25, pp. 203-8, 2007, ().
@article{Knudsen2007,
title = {Use of modeling to evaluate the cost-effectiveness of cancer screening programs},
author = {Amy Knudsen and Pamela M. McMahon and G. Scott Gazelle},
url = {http://www.ncbi.nlm.nih.gov/pubmed/17210941},
year = {2007},
date = {2007-01-01},
urldate = {2007-01-01},
journal = {J Clin Oncol},
volume = {25},
pages = {203-8},
abstract = {Cost-effectiveness analysis (CEA) is an analytic tool that provides
a framework for comparing the health benefits and resource expenditures
associated with competing medical and public health interventions,
thereby allowing decision makers to identify interventions that yield
the greatest amount of health, given their resource constraints.
Models are important components of most, if not all, CEAs, and they
play a key role in evaluating the cost-effectiveness of cancer screening
programs, in particular. In this article, we describe the basic types
of models used to evaluate cancer screening programs and provide
examples of the use of models in CEAs and to guide cancer screening
policy. Finally, we offer some suggestions for important concepts
to consider when interpreting model results.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
a framework for comparing the health benefits and resource expenditures
associated with competing medical and public health interventions,
thereby allowing decision makers to identify interventions that yield
the greatest amount of health, given their resource constraints.
Models are important components of most, if not all, CEAs, and they
play a key role in evaluating the cost-effectiveness of cancer screening
programs, in particular. In this article, we describe the basic types
of models used to evaluate cancer screening programs and provide
examples of the use of models in CEAs and to guide cancer screening
policy. Finally, we offer some suggestions for important concepts
to consider when interpreting model results.



